Effective Categorization of Tolerance to Salt Stress through Clustering Prunus Rootstocks According to Their Physiological Performances
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material and Salt Stress Treatment
2.2. Root Length Measurement
2.3. Root Respiration Rate
2.4. Malondialdehyde Determination
2.5. Leaf Gas Exchange Measurements
2.6. Statistical Analysis
3. Results
3.1. Root Phenotype in Prunus spp. Rootstocks under Salt Stress
3.2. Malondialdehyde (MDA) Content in Roots
3.3. Root Respiration Rate
3.4. Gas Exchange Parameters
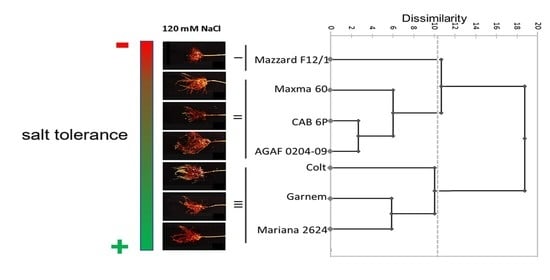
3.5. Salt Tolerance Coefficients and Cluster Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. FAO Land and Plant Nutrition Management Service. 2008. Available online: http://www.fao.org/ag/agl/agll/spush (accessed on 5 February 2021).
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. Gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 2011, 30, 435–458. [Google Scholar] [CrossRef]
- Brown, G.E., Jr. Research Databases. Bibliography on Salt Tolerance. USDA-ARS US Dep. Agric. Res. Serv. Riverside CA 2008. Available online: https://www.ars.usda.gov/pacific-west-area/riverside-ca/agricultural-water-efficiency-and-salinity-research-unit/docs/research-databases/ (accessed on 21 December 2020).
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef]
- Munns, R. Physiological processes limiting plant growth in saline soils: Some dogmas and hypotheses. stress. Plant Cell Environ. 1993, 16, 15–24. [Google Scholar] [CrossRef]
- Yu, J.; Chen, S.; Zhao, Q.; Wang, T.; Yang, C.; Diaz, C.; Sun, G.; Dai, S. Physiological and proteomic analysis of salinity tolerance in Puccinellia tenuiflora. J. Proteome Res. 2011, 10, 3852–3870. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Guo, S.R.; Sun, J.; Yuan, L.Y. Effects of salt stress on the structure and function of the photosynthetic apparatus in Cucumis sativus and its protection by exogenous putrescine. Physiol. Plant. 2012, 146, 285–296. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Kotuby-Amacher, J.; Koenig, R.; Kitchen, B. Salinity and Plant Tolerance; Utah State University Extension: Logan, UT, USA, 2000; AG-SO-03; Available online: https://digitalcommons.usu.edu/cgi/viewcontent.cgi?article=1042&context=extension_histall (accessed on 12 December 2020).
- Byrne, P.F.; Volk, G.M.; Gardner, C.; Gore, M.A.; Simon, P.W.; Smith, S. Sustaining the future of plant breeding: The critical role of the USDA-ARS National Plant Germplasm System. Crop Sci. 2018, 58, 451–468. [Google Scholar] [CrossRef] [Green Version]
- Sansavini, S.; Lugli, S. Sweet cherry breeding programs in Europe and Asia. Acta Hortic. 2008, 795, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Sawamura, Y.; Suesada, Y.; Sugiura, T.; Yaegaki, H. Chilling requirements and blooming dates of leading peach cultivars and a promising early maturing peach selection, Momo Tsukuba 127. Hortic. J. 2017, 86, 426–436. [Google Scholar] [CrossRef] [Green Version]
- Apey, A. La Fruticultura en Chile: Tendencias Productivas y su Expresión Territorial. 2019. Available online: https://www.odepa.gob.cl/publicaciones/articulos/la-fruticultura-en-chile-tendencias-productivas-y-su-expresion-territorial (accessed on 26 February 2021).
- Ikinci, A.; Bolat, I.; Ercisli, S.; Kodad, O. Influence of rootstocks on growth, yield, fruit quality and leaf mineral element contents of pear cv. ‘Santa Maria’ in semi-arid conditions. Biol. Res. 2014, 47, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Marguerit, E.; Brendel, O.; Lebon, E.; van Leeuwen, C.; Ollat, N. Rootstock control of scion transpiration and its acclimation to water deficit are controlled by different genes. New Phytol. 2012, 194, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Peccoux, A.; Loveys, B.; Zhu, J.; Gambetta, G.A.; Delrot, S.; Vivin, P.; Schultz, H.R.; Ollat, N.; Dai, Z. Dissecting the rootstock control of scion transpiration using model-assisted analyses in grapevine. Tree Physiol. 2018, 38, 1026–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, F. Recent advances in research on Japanese pear rootstocks. J. Jpn. Soc. Hortic. Sci. 2012, 81, 1–10. [Google Scholar] [CrossRef]
- Bernstein, N.; Meiri, A.; Zilberstaine, M. Root growth of avocado is more sensitive to salinity than shoot growth. J. Am. Soc. Hortic. Sci. 2004, 129, 188–192. [Google Scholar] [CrossRef]
- Rewald, B.; Leuschner, C.; Wiesman, Z.; Ephrath, J.E. Influence of salinity on root hydraulic properties of three olive varieties. Plant Biosyst. 2011, 145, 12–22. [Google Scholar] [CrossRef]
- Andreu, P.; Arbeloa, A.; Lorente, P.; Marín, J.A. Early detection of salt stress tolerance of Prunus rootstocks by excised root culture. HortScience 2011, 46, 80–85. [Google Scholar] [CrossRef]
- Papadakis, I.E.; Veneti, G.; Chatzissavvidis, C.; Therios, I. Physiological and growth responses of sour cherry (Prunus cerasus L.) plants subjected to short-term salinity stress. Acta Bot. Croat. 2018, 77, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Tanou, G.; Molassiotis, A.; Diamantidis, G. Hydrogen peroxide-and nitric oxide-induced systemic antioxidant prime-like activity under NaCl-stress and stress-free conditions in citrus plants. J. Plant Physiol. 2009, 166, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- AbdElgawad, H.; Zinta, G.; Hegab, M.; Pandey, R.; Asard, H.; Abuelsoud, W. High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front. Plant Sci. 2016, 7, 276. [Google Scholar] [CrossRef] [Green Version]
- Imlay, J.A.; Linn, S. DNA damage and oxygen radical toxicity. Science 1988, 240, 1302–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Pitzschke, A.; Forzani, C.C.; Hirt, H. Reactive oxygen species signaling in plants. Antiox. Redox Signal. 2006, 8, 1757–1764. [Google Scholar] [CrossRef]
- Katsuhara, M.; Otsuka, T.; Ezaki, B. Salt stress-induced lipid peroxidation is reduced by glutathione S-transferase, but this reduction of lipid peroxides is not enough for a recovery of root growth in Arabidopsis. Plant Sci. 2005, 69, 369–373. [Google Scholar] [CrossRef]
- Maia, J.M.; Voigt, E.L.; Macêdo, C.E.C.; Ferreira-Silva, S.L.; Silveira, J.A.G. Salt-induced changes in antioxidative enzyme activities in root tissues do not account for the differential salt tolerance of two cowpea cultivars. Braz. J. Plant Physiol. 2010, 22, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Khataar, M.; Mohammadi, M.; Shabani, F. Soil salinity and matric potential interaction on water use, water use efficiency and yield response factor of bean and wheat. Sci. Rep. 2018, 8, 2679. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H. Respiration associated with growth, maintenance, and ion uptake. In Plant Physiological Ecology; Lambers, H., Chapin, F., Pons, T.L., Eds.; Springer: New York, NY, USA, 2008; pp. 134–140. [Google Scholar]
- Moya, J.L.; Primo-Millo, E.; Talon, M. Morphological factors determining salt tolerance in citrus seedlings: The shoot to root ratio modulates passive root uptake of chloride ions and their accumulation in leaves. Plant Cell Environ. 1999, 22, 1425–1433. [Google Scholar] [CrossRef]
- Rajendran, K.; Tester, M.; Roy, S.J. Quantifying the three maincomponents of salinity tolerance in cereals. Plant Cell Environ. 2009, 32, 237. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Motaium, R.; Hu, H.; Brown, P.H. The relative tolerance of six Prunus rootstocks to boron and salinity. J. Am. Soc. Hortic. Sci. 1994, 119, 1169–1175. [Google Scholar] [CrossRef]
- Rieger, M. Salt stress resistance of peach and four north american Prunus species. Acta Hortic. 2001, 557, 181–192. [Google Scholar] [CrossRef]
- Ranjbarfordoei, A.; Samson, R.; Van Damme, P. Chlorophyll fluorescence performance of sweet almond (Prunus dulcis (Miller) D. Webb) in response to salinity stress induced by NaCl. Photosynthetica 2006, 44, 513–522. [Google Scholar] [CrossRef]
- Najafian, S.; Rahemi, M.; Tavallali, V. Effect of salinity on tolerance of two bitter almond rootstocks. Am.-Eurasian J. Agri. Environ. Sci. 2008, 3, 264–268. [Google Scholar]
- Küçükyumuk, C.; Yildiz, H.; Küçükyumuk, Z.; Ünlükara, A. Responses of ‘0900 Ziraat’ sweet cherry variety grafted on different rootstocks to salt stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Zrig, A.; Mohamed, H.B.; Tounekti, T.; Ennajeh, M.; Valero, D.; Khemira, H. A comparative study of salt tolerance of three almond rootstocks: Contribution of organic and inorganic solutes to osmotic adjustment. J. Agr. Sci. Tech. 2015, 17, 675–689. [Google Scholar]
- Wang, X.M.; Huang, T.; Wu, W.L.; Li, W.L.; Zhu, H. Effects of salt stress on photosynthetic characteristics of beach plum and other Prunus species. Acta Hortic. 2016, 1112, 233–240. [Google Scholar]
- Arbona, V.; Hossain, Z.; Lopez-Climent, M.F.; Perez-Clemente, R.M.; Gomez-Cadenas, A. Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiol. Plant. 2008, 132, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, P.; Almada, R.D.; Salvatierra, A.; Toro, G.; Arismendi, M.J.; Pino, M.T.; Sagredo, B.; Pinto, M. Physiological and morphological responses of Prunus species with different degree of tolerance to long-term root hypoxia. Sci. Hortic. 2014, 180, 14–23. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops–what is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Gainza, F.; Opazo, I.; Guajardo, V.; Meza, P.; Ortiz, M.; Pinochet, J.; Muñoz, C. Rootstock breeding in Prunus species: Ongoing efforts and new challenges. Chil. J. Agric. Res. 2015, 75, 6–16. [Google Scholar] [CrossRef] [Green Version]
- Nimbolkar, P.K.; Awachare, C.; Reddy, Y.T.N.; Chander, S.; Hussain, F. Role of rootstocks in fruit production–A Review. J. Agric. Eng. Food Technol. 2016, 3, 183–188. [Google Scholar]
- Shahzad, A.; Ahmad, M.; Iqbal, M.; Ahmed, I.; Ali, G. Evaluation of wheat landrace genotypes for salinity tolerance at vegetative stage by using morphological and molecular markers. Genet. Mol. Res. 2012, 11, 679–692. [Google Scholar] [CrossRef]
- Cuiyu, L.; Ming, Y.; Xianbin, H.; Zhaohe, Y. Effects of NaCl stress on growth and ion homeostasis in pomegranate tissues. Eur. J. Hortic. Sci. 2020, 85, 42–50. [Google Scholar] [CrossRef]
- Askri, H.; Daldoul, S.; Ammar, A.B.; Rejeb, S.; Jardak, R.; Rejeb, M.N.; Mliki, A.; Ghorbel, A. Short-term response of wild grapevines (Vitis vinifera L. ssp. sylvestris) to NaCl salinity exposure: Changes of some physiological and molecular characteristics. Acta Physiol. Plant. 2012, 34, 957–968. [Google Scholar] [CrossRef]
- Bonomelli, C.; Celis, V.; Lombardi, G.; Mártiz, J. Salt stress effects on avocado (Persea americana Mill.) plants with and without seaweed extract (Ascophyllum nodosum) application. Agronomy 2018, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Ben-Gal, J.; Shtein, I.; Bustan, A.; Dag, A.; Erel, R. Root structural plasticity enhances salt tolerance in mature olives. Environ. Exp. Bot. 2020, 179, 104224. [Google Scholar] [CrossRef]
- Picchioni, G.A.; Miyamoto, S.; Storey, J.B. Salt effects on growth and ion uptake of pistachio rootstock seedlings. J. Am. Soc. Hortic. Sci. 1990, 115, 647–653. [Google Scholar] [CrossRef]
- Rahneshan, Z.; Nasibi, F.; Moghadam, A.H. Effects of salinity stress on some growth, physiological, biochemical parameters and nutrients in two pistachio (Pistacia vera L.) rootstocks. J. Plant Interact. 2018, 13, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Massai, R.; Remorini, D.; Tattini, M. Gas exchange, water relations and osmotic adjustment in two scion/rootstock combinations of Prunus under various salinity concentrations. Plant Soil 2004, 259, 153–162. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Al Kharusi, L.; Al Yahyai, R.; Yaish, M.W. Antioxidant response to salinity in Salt-Tolerant and Salt-Susceptible cultivars of date palm. Agriculture 2019, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Jacoby, R.P.; Taylor, N.L.; Millar, A.H. The role of mitochondrial respiration in salinity tolerance. Trends Plant Sci. 2011, 16, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.; Epstein, E. Varietal differences in salt-induced respiration in barley. Plant Sci. Lett. 1984, 35, 1–3. [Google Scholar] [CrossRef]
- Epron, D.; Toussaint, M.-L.; Badot, P.-M. Effects of sodium chloride salinity on root growth and respiration in oak seedlings. Ann. For. Sci. 1999, 56, 41–47. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Morris, J.T. Whole-plant gas exchange responses of Spartina alterniflora (Poaceae) to a range of constant and transient salinities. Am. J. Bot. 1994, 81, 659–665. [Google Scholar] [CrossRef]
- Rachmilevitch, S.; Lambers, H.; Huang, B. Root respiratory characteristics associated with plant adaptation to high soil temperature for geothermal and turf-type Agrostis species. J. Exp. Bot. 2006, 57, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, M.; Gale, J. Maintenance respiration and carbon balance of plants at low levels of sodium chloride salinity. J. Exp. Bot. 1981, 32, 933–5941. [Google Scholar] [CrossRef]
- Karaba, A.; Dixit, S.; Greco, R.; Aharoni, A.; Trijatmiko, K.R.; Marsch-Martinez, N.; Krishnan, A.; Nataraja, K.N.; Udayakumar, M.; Pereira, A. Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene. Proc. Natl. Acad. Sci. USA 2007, 104, 15270–15275. [Google Scholar] [CrossRef] [Green Version]
- Omamt, E.N.; Hammes, P.S.; Robbertse, P.J. Differences in salinity tolerance for growth and water-use efficiency in some amaranth (Amaranthus spp.) genotypes. N. Z. J. Crop Hortic. Sci. 2006, 34, 11–22. [Google Scholar] [CrossRef]
- Ziska, L.H.; Seemann, J.R.; DeJong, T.M. Salinity induced limitations on photosynthesis in Prunus salicina, a deciduous tree species. Plant Physiol. 1990, 93, 864–870. [Google Scholar] [CrossRef] [Green Version]
- Meloni, D.A.; Oliva, M.A.; Martinez, C.A.; Cambraia, J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003, 49, 69–76. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Rea, E.; Colla, G. Improving melon and cucumber photosynthetic activity, mineral composition, and growth performance under salinity stress by grafting onto Cucurbita hybrid rootstocks. Photosynthetica 2012, 50, 180–188. [Google Scholar] [CrossRef]
- Tomás, M.; Medrano, H.; Escalona, J.M.; Martorell, S.; Pou, A.; Ribas-Carbó, M.; Flexas, J. Variability of water use efficiency in grapevines. Environ. Exp. Bot. 2014, 103, 148–157. [Google Scholar] [CrossRef]
- Yin, R.; Bai, T.; Ma, F.; Wang, X.; Li, Y.; Yue, Z. Physiological responses and relative tolerance by Chinese apple rootstocks to NaCl stress. Sci. Hortic. 2010, 126, 247–252. [Google Scholar] [CrossRef]
- Ganopoulos, I.; Tourvas, N.; Xanthopoulou, A.; Aravanopoulos, F.A.; Avramidou, E.; Zambounis, A.; Tsaftaris, A.; Madesis, P.; Sotiropoulos, T.; Koutinas, N. Phenotypic and molecular characterization of apple (Malus × domestica Borkh) genetic resources in Greece. Sci. Agric. 2018, 75, 509–518. [Google Scholar] [CrossRef] [Green Version]
- Goharrizi, K.J.; Baghizadeh, A.; Kalantar, M.; Fatehi, F. Combined effects of salinity and drought on physiological and biochemical characteristics of pistachio rootstocks. Sci. Hortic. 2020, 261, 108970. [Google Scholar] [CrossRef]







| Maximum Root Length (cm) | ||||||
|---|---|---|---|---|---|---|
| NaCl Solution Concentration | ||||||
| 0 mM | 60 mM | 120 mM | ||||
| Mariana 2624 | 23.4 a | ±1.9 | 16.6 b | ±3.7 | 13.9 b | ±1.7 |
| Cab 6P | 20.2 a | ±3.0 | 17.0 a | ±1.1 | 15.0 b | ±0.8 |
| Agaf 0204-09 | 27.4 a | ±1.8 | 23.0 b | ±1.3 | 21.9 b | ±1.3 |
| Maxma60 | 27.8 a | ±2.9 | 13.7 b | ±2.8 | 14.4 b | ±1.6 |
| Garnem | 19.6 a | ±2.0 | 14.7 b | ±1.0 | 12.4 b | ±2.4 |
| Colt | 21.0 a | ±1.6 | 17.0 b | ±0.8 | 15.1 c | ±0.4 |
| Mazzard F12/1 | 19.1 a | ±1.8 | 13.9 b | ±1.8 | 8.5 c | ±2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toro, G.; Pimentel, P.; Salvatierra, A. Effective Categorization of Tolerance to Salt Stress through Clustering Prunus Rootstocks According to Their Physiological Performances. Horticulturae 2021, 7, 542. https://doi.org/10.3390/horticulturae7120542
Toro G, Pimentel P, Salvatierra A. Effective Categorization of Tolerance to Salt Stress through Clustering Prunus Rootstocks According to Their Physiological Performances. Horticulturae. 2021; 7(12):542. https://doi.org/10.3390/horticulturae7120542
Chicago/Turabian StyleToro, Guillermo, Paula Pimentel, and Ariel Salvatierra. 2021. "Effective Categorization of Tolerance to Salt Stress through Clustering Prunus Rootstocks According to Their Physiological Performances" Horticulturae 7, no. 12: 542. https://doi.org/10.3390/horticulturae7120542
APA StyleToro, G., Pimentel, P., & Salvatierra, A. (2021). Effective Categorization of Tolerance to Salt Stress through Clustering Prunus Rootstocks According to Their Physiological Performances. Horticulturae, 7(12), 542. https://doi.org/10.3390/horticulturae7120542