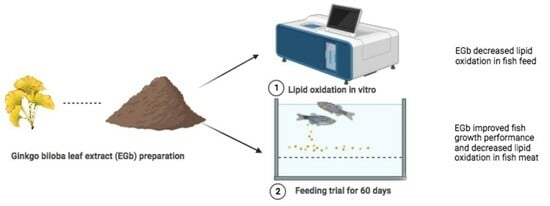
Extract of Ginkgo biloba Leaves (EGb) Decrease Lipid Oxidation in Fish Feed and Meat and Enhance Growth and Antioxidant Capacity in Jian Carp (Cyprinus carpio var. Jian)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagent
2.2. EGbs Preparation and Composition Analyses
2.3. Measurement of Flavonoids Content, Total Antioxidant Capacity and Metal-Chelating Ability
2.4. Measurement of Lipid Oxidation in Linoleic Acid and Linolenic Acid Emulsion
2.5. Measurement of Lipid Oxidation in Fish Feed
2.6. Feeding Trial
2.7. Biochemical Analysis
2.8. Statistical Analysis
3. Results
3.1. Flavonoids Content, Total Antioxidant Capacity and Metal-Chelating Ability in EGbs
3.2. Effects of EGbs on the Lipid Oxidation in Linoleic Acid and Linolenic Acid
3.3. The EAE Composition
3.4. The Effects of EAE on the Lipid Oxidation in Fish Feed
3.5. Effects of Dietary EAE on Fish Growth Performance
3.6. Effects of Dietary EAE on the Biochemical Parameters in Hepatopancreas of Jian Carp
3.7. Effects of Dietary EAE on the Biochemical Parameters in Intestine of Jian Carp
3.8. Effects of EAE on the Biochemical Parameters in Gills of Jian Carp
3.9. Effects of EAE on the Biochemical Parameters in Plasma and Erythrocytes of Jian Carp
3.10. Effects of EAE on the Biochemical Parameters in Meat of Jian Carp
3.11. Effects of Dietary EAE on the Hot-Drying-Induced Lipid Oxidation in Meat of Jian Carp
4. Discussion
4.1. EGb Inhibited the Lipid Oxidation in Fish Feed
4.2. Dietary EAE Supplementation Improved Fish Growth Performance
4.3. Dietary EAE Decreased Lipid Oxidation in Fish Meat through Inhibiting the Induction Effect of Hemoglobin in Erythrocyte
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Godwin, A.; Prabhu, H.R. Lipid peroxidation of fish oils. Indian. J. Clin. Biochem. 2006, 21, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Chen, S.N.; Cao, J.Y.; Zhou, J.Y.; Chen, Y.Z.; Jamali, M.A.; Zhang, Y.W. Hydrolysis and oxidation of protein and lipids in dry-salted grass carp (Ctenopharyngodon idella) as affected by partial substitution of NaCl with KCl and amino acids. RSC Adv. 2019, 9, 39545–39560. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; García García, B.; Jordán, M.J.; Hernández, M.D. Natural antioxidants in extruded fish feed: Protection at different storage temperatures. Anim. Feed. Sci. Technol. 2014, 195, 112–119. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S. Effect of bleeding on lipid oxidation and quality changes of Asian seabass (Lates calcarifer) muscle during iced storage. Food. Chem. 2011, 124, 459–467. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S.; Kamal-Eldin, A. Haemoglobin-mediated lipid oxidation in the fish muscle: A review. Trends Food Sci. Technol. 2012, 28, 33–43. [Google Scholar] [CrossRef]
- Błaszczyk, A.; Augustyniak, A.; Skolimowski, J. Ethoxyquin: An Antioxidant Used in Animal Feed. Int. J. Food Sci. 2013, 1–12. [Google Scholar] [CrossRef]
- Song, C.; Liu, B.; Xu, P.; Xie, J.; Ge, X.; Zhou, Q.; Sun, C.; Zhang, H.; Shan, F.; Yang, Z. Oxidized fish oil injury stress in Megalobrama amblycephala: Evaluated by growth, intestinal physiology, and transcriptome-based PI3K-Akt/NF-κB/TCR inflammatory signaling. Fish Shellfish Immunol. 2018, 81, 446–455. [Google Scholar]
- Li, H.; Zhou, X.; Gao, P.; Li, Q.; Li, H.; Huang, R.; Wu, M. Inhibition of lipid oxidation in foods and feeds and hydroxyl radical-treated fish erythrocytes: A comparative study of Ginkgo biloba leaves extracts and synthetic antioxidants. Anim. Nutr. 2016, 2, 234–241. [Google Scholar] [CrossRef]
- Ito, N.; Fukushima, S.; Tsuda, H. Carcinogenicity and modification of the carcinogenic response by BHA, BHT, and other antioxidants. Crit. Rev. Toxicol. 1985, 15, 109–150. [Google Scholar]
- EFSA. Safety and efficacy of ethoxyquin (6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline) for all animal species. EFSA J. 2015, 13, 4272. [Google Scholar]
- Aksoy, L.; Kolay, E.; Agilonu, Y.; Aslan, Z.; Kargioglu, M. Free radical scavenging activity, total phenolic content, total antioxidant status, and total oxidant status of endemic Thermopsis turcica. Saudi J. Biol. Sci. 2013, 20, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lou, K.; Wu, G.; Wu, X.; Zhou, X.; Feng, Y.; Zhang, H.; Yu, P. Bioactive metabolites in of Ginkgo biloba leaves: Variations by seasonal, meteorological and soil. Braz. J. Biol. 2019, 80, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Sun, Z.; Liu, Q.; Ye, H.; Zou, C.; Ye, C.; Wang, A.; Lin, H. Effects of dietary Ginkgo biloba leaf extract on growth performance, plasma biochemical parameters, fish composition, immune responses, liver histology, and immune and apoptosis-related genes expression of hybrid grouper (Epinephelus lanceolatus♂× Epinephelus fuscoguttatus♀) fed high lipid diets. Fish Shellfish Immun. 2018, 72, 399–409. [Google Scholar]
- Mahadevan, S.; Park, Y. Multifaceted therapeutic benefifits of Ginkgo biloba L.: Chemistry, effificacy, safety, and uses. J. Food. Sci. 2008, 73, R14e9. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.F.; Feng, L.; Kuang, S.Y.; Liu, Y.; Jiang, J.; Hu, K.; Jiang, W.D.; Li, S.H.; Tang, L.; Zhou, X.Q. Effect of dietary arginine on growth, intestinal enzyme activities and gene expression in muscle, hepatopancreas and intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Br. J. Nutr. 2012, 108, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Long, J.; Li, H.; Xu, J.; Yuan, J.; Yang, Q.; Feng, L.; Wu, M.; Jiang, J. The Protective Effect of a Dietary Extract of Mulberry (Morus alba L.) Leaves against a High Stocking Density, Copper and Trichlorfon in Crucian Carp (Carassius auratus). Animals 2023, 13, 2652. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.P.; Lu, Y.H.; Wei, D.Z. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J. Agric. Food. Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Zhao, J.; Posa, D.K.; Kumar, V.; Hoetker, D.; Kumar, A.; Ganesan, S.; Riggs, D.W.; Bhatnagar, A.; Wempe, M.F.; Baba, S.P. Carnosine protects cardiac myocytes against lipid peroxidation products. Amino Acids 2018, 51, 123–128. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Bone, D.E.; Carrington, M.F. Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem. 2005, 91, 485–494. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S. Comparative studies of four different phenolic compounds on in vitro antioxidative activity and the preventive effect on lipid oxidation of fish oil emulsion and fish mince. Food Chem. 2010, 119, 123–132. [Google Scholar] [CrossRef]
- Chen, G.; Wu, M.; Li, H.; Xu, J.; Liu, H.; Du, W.; Yang, Q.; Feng, L.; Jiang, J. Scoparia dulcis L. Extract Relieved High Stocking Density-Induced Stress in Crucian Carp (Carassius auratus). Animals 2023, 13, 2522. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Wu, M.; Wang, J.; Qin, C.J.; Long, J.; Zhou, S.S.; Yuan, P.; Jing, X.Q. Protective role of Angelica sinensis extract on trichlorfon-induced oxidative damage and apoptosis in gills and erythrocytes of fish. Aquaculture 2020, 519, 734895. [Google Scholar] [CrossRef]
- Li, H.T.; Feng, L.; Jiang, W.D.; Liu, Y.; Jiang, J.; Li, S.H.; Zhou, X.Q. Oxidative stress parameters and anti-apoptotic response to hydroxyl radicals in fish erythrocytes: Protective effects of glutamine, alanine, citrulline and proline. Aquat. Toxicol. 2013, 126, 169–179. [Google Scholar] [CrossRef]
- Jiang, W.D.; Wu, P.; Kuang, S.Y.; Liu, Y.; Jiang, J.; Hu, K.; Li, S.H.; Tang, L.; Feng, L.; Zhou, X.Q. Myo-inositol prevents copper-induced oxidative damage and changes in antioxidant capacity in various organs and the enterocytes of juvenile Jian carp (Cyprinus carpio var. Jian). Aquat. Toxicol. 2011, 105, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Meng, Y.; Zhao, X.; Fan, W.; Yi, T.; Wang, X. Sunflower oil flavored by essential oil from Punica granatum cv. Heyinshiliu peels improved its oxidative stability and sensory properties. LWT 2019, 111, 55–61. [Google Scholar] [CrossRef]
- Recknagel, R.O.; Glende, E.A. Spectrophotometric detection of lipid conjugated dienes. Method Enzymol. 1984, 105, 331–337. [Google Scholar]
- Martínez-Yusta, A.; Goicoechea, E.; Guillén, M.D. A review of thermo-oxidative degradation of food lipids studied by 1HNMR spectroscopy: Influence of degradative conditions and food lipid nature. Compr. Rev. Food Sci. F 2014, 13, 838–859. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Zu, Y.G.; Yang, L.; Lu, Q.; Wang, W. Antioxidant effects of rosemary extracts on sunflower oil compared with synthetic antioxidants. Int. J. Food Sci. Technol. 2014, 49, 385–391. [Google Scholar] [CrossRef]
- El-Husseiny, O.M.; Abdul-Aziz, G.M.; Goda, A.S.; Suloma, A. Effect of altering linoleic acid and linolenic acid dietary levels and ratios on the performance and tissue fatty acid profiles of Nile tilapia Oreochromis niloticus fry. Aquac. Int. 2010, 18, 1105–1119. [Google Scholar] [CrossRef]
- Wei, X.B.; Liu, H.Q.; Sun, X.; Fu, F.; Zhang, X.; Wang, J.; An, J.; Ding, H. Hydroxysafflor yellow A protects rat brains against ischemia-reperfusion injury by antioxidant action. Neurosci. Lett. 2005, 386, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, S.; Duan, X.; Feng, X.; Yang, Y. The interaction effects of coke oven emissions exposure and metabolic enzyme Gene variants on total antioxidant capacity of workers. Environ. Toxicol. Pharmacol. 2019, 70, 103197. [Google Scholar] [CrossRef] [PubMed]
- Lugasi, A.; Dworschak, E.; Horvatovich, P. Additional information to the in vitro antioxidant activity of Ginkgo biloba L. Phytother. Res. 1999, 13, 160–162. [Google Scholar] [CrossRef]
- Zhao, L.J.; Liu, W.; Xiong, S.H.; Tang, J.; Lou, Z.H.; Xie, M.X.; Xia, B.H.; Lin, L.M.; Liao, D.F. Determination of total flavonoids contents and antioxidant activity of Ginkgo biloba leaf by near-infrared reflectance method. Int. J. Anal. Chem. 2018, 8195784. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.K.; Jung, H.A.; Chung, H.Y.; Choi, J.S. In vitro peroxynitrite scavenging activity of 6-hydroxykynurenic acid and other flavonoids from Gingko biloba yellow leaves. Arch. Pharm. Res. 2006, 29, 1074–1079. [Google Scholar] [CrossRef]
- Ni, Y.; Duan, Z.; Zhou, D.; Liu, S.; Wan, H.; Gui, C.; Zhang, H. Identification of Structural Features for the Inhibition of OAT3-Mediated Uptake of Enalaprilat by Selected Drugs and Flavonoids. Front. Pharmacol. 2020, 11, 802. [Google Scholar] [CrossRef]
- Fuhrman, B.; Aviram, M. Flavonoids protect LDL from oxidation and attenuate atherosclerosis. Curr. Opin. Lipidol. 2001, 12, 41–48. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Chan, P.T.; Ho, K.Y.; Fung, K.P.; Wang, J. Antioxidant activity of natural flavonoids is governed by number and location of their aromatic hydroxyl groups. Chem. Phys. Lipids 1996, 79, 157–163. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Rather, A.A. Biochemical responses induced by sub lethal concentrations of carbaryl and parathion on certain enzymes of fresh water catfish Clarias batrachus. Int. J. Biol. Sci. 2015, 4, 52–56. [Google Scholar]
- Dikshith, T.S.; Datta, K.; Kushwah, H.; Raizada, R. Histopathological and biochemical changes in guinea pigs after repeated dermal exposure to benzene hexachloride. Toxicology 1978, 10, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Johari, S.A.; Sarkheil, M.; Asghari, S.; Haghighat, F.; Dekani, L.; Keyvanshokooh, S. Comparative toxicity of nanoparticulate and ionic copper following dietary exposure to common carp (Cyprinus carpio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 229, 108680. [Google Scholar] [CrossRef] [PubMed]
- Ahmadifar, E.; Yousefi, M.; Karimi, M.; Fadaei Raieni, R.; Dadar, M.; Yilmaz, S.; Dawood, M.; Abdel-Latif, H. Benefits of Dietary Polyphenols and Polyphenol-Rich Additives to Aquatic Animal Health: An Overview. Revi. Fish. Sci. Aquac. 2021, 29, 478–511. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.; Hendam, B.M.; Nofal, M.I.; El-Son, M.A. Ginkgo biloba leaf extract improves growth, intestinal histomorphometry, immunity, antioxidant status and modulates transcription of cytokine genes in hapa-reared Oreochromis niloticus. Fish Shellfish Immunol. 2021, 117, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Wang, F.; Huang, L.; Liu, C.; Dong, W.; Zhuang, X.; Yin, X.; Liu, Y.; Wang, W. Effects of dietary Ginkgo biloba leaf extract on growth performance, immunity and environmental stress tolerance of Penaeus vannamei. Fish Shellfish Immunol. 2023, 132, 108500. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, W.; Lan, T.; Zhang, X. Protective effects of extract of Ginkgo biloba on adriamycin-induced heart failure and its mechanism: Role of ghrelin peptide. Zhongguo Zhong Yao Za Zhi 2009, 34, 2786–2789. [Google Scholar]
- Shi, L.; Du, X.; Jiang, H.; Xie, J. Ghrelin and neurodegenerative disorders—A review. Mol. Neurobiol. 2017, 54, 1144–1155. [Google Scholar] [CrossRef]
- Gilloteaux, J.; Kashouty, R.; Yono, N. The perinuclear space of pancreatic acinar cells and the synthetic pathway of zymogen in Scorpaena scrofa L.: Ultrastructural aspects. Tissue Cell 2008, 40, 7–20. [Google Scholar] [CrossRef]
- Zambonino Infante, J.L.; Cahu, C.L. Ontogeny of the gastrointestinal tract of marine fish larvae. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 130, 477–487. [Google Scholar] [CrossRef]
- Geering, K. Subunit assembly and functional maturation of Na, K-ATPase. J. Membr. Biol. 1990, 115, 109–121. [Google Scholar] [CrossRef]
- Suzer, C.; Aktülün, S.; Çoban, D.; Okan Kamacı, H.; Saka, Ş.; Fırat, K.; Alpbaz, A. Digestive enzyme activities in larvae of sharpsnout seabream (Diplodus puntazzo). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhao, S.; Chen, G.; Jiang, W.; Liu, Y.; Jiang, J.; Hu, K.; Li, S.; Zhou, X. Antioxidant status of serum, muscle, intestine and hepatopancreas for fish fed graded levels of biotin. Fish Physiol. Biochem. 2013, 40, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Gliszczyńska-Świgło, A. Antioxidant activity of water soluble vitamins in the TEAC (trolox equivalent antioxidant capacity) and the FRAP (ferric reducing antioxidant power) assays. Food Chem. 2006, 96, 131–136. [Google Scholar] [CrossRef]
- Kudolo, G.B.; Delaney, D.; Blodgett, J. Short-term oral ingestion of Ginkgo biloba extract (EGb 761) reduces malondialdehyde levels in washed platelets of type 2 diabetic subjects. Diabetes Res. Clin. Pract. 2005, 68, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Gong, Z.; Li, J.; Li, X. Ginkgo biloba extract protects against alcohol-induced liver injury in rats. Phytother. Res. 2007, 21, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Chen, J.; Ding, Y.; Cheng, J.; Yang, S.; Ding, Z.; Dai, Q.; Ding, Z. In vitro antioxidant and immunostimulating activities of polysaccharides from Ginkgo biloba leaves. Int. J. Biol. Macromol. 2019, 124, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.X.; He, W.; Rinne, T.; Liu, Y.; Zhao, M.Q.; Wu, W.K. The effect of Ginkgo biloba extract (EGb 761) pretreatment on intestinal epithelial apoptosis induced by intestinal ischemia/reperfusion in rats: Role of ceramide. Am. J. Chin. Med. 2007, 35, 805–819. [Google Scholar] [CrossRef]
- Seyoum, A.; Asres, K.; El-Fiky, F.K. Structure–radical scavenging activity relationships of flavonoids. Phytochemistry 2006, 67, 2058–2070. [Google Scholar] [CrossRef]
- Yokomizo, A.; Moriwaki, M. Effects of uptake of flavonoids on oxidative stress induced by hydrogen peroxide in human intestinal Caco-2 cells. Biosci. Biotechnol. Biochem. 2006, 70, 1317–1324. [Google Scholar] [CrossRef]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant defenses in fish: Biotic and abiotic factors. Rev. Fish Biol. Fisher. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Ni, M.; Liu, M.; Lou, J.; Mi, G.; Gu, Z. Stocking density alters growth performance, serum biochemistry, digestive enzymes, immune response, and muscle quality of largemouth bass (Micropterus salmoides) in in-pond raceway system. Fish Physiol. Biochem. 2021, 47, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Das, S. Deltamethrin Induced Alteration of Biochemical Parameters in Channa punctata, Bloch and its Amelioration by Quercetin. Bull. Environ. Contam. Toxicol. 2017, 98, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Kirshenbaum, L.A.; Singal, P.K. Increase in endogenous antioxidant enzymes protects hearts against reperfusion injury. Am. J. Physiol. 1993, 265, H484–H492. [Google Scholar] [CrossRef]
- Yim, T.K.; Wu, W.K.; Pak, W.F.; Mak, D.H.F.; Ko, K.M. Myocardial protection against ischaemia- reperfusion injury by a Polygonum multiflorum extract supplemented ‘Dang-Gui decoction for enriching blood’, a compound formulation, ex vivo. Phytother. Res. 2000, 14, 195–199. [Google Scholar] [CrossRef]
- Reed, D.J. Glutathione: Toxicological implications. Annu. Rev. Pharm. Toxicol. 1990, 30, 603–631. [Google Scholar] [CrossRef]
- Sugihara, N.; Tsuruta, Y.; Date, Y.; Furuno, K.; Kohashi, K. High peroxidative susceptibility of fish oil polyunsaturated fatty acid in cultured rat hepatocytes. Toxicol. Appl. Pharmacol. 1994, 126, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Liu, N.; Sun, Y.; Sun, G.; Wang, S.; Yang, M.; Yang, M.; Wang, X.; Zhou, D.; Ge, Y.; et al. Effect of drying processes on the occurrence of lipid oxidation-derived 4-hydroxy-2-hexenal and 4-hydroxy-2-nonenal in Spanish mackerel (Scomberomorus niphonius). Food Sci. Nutri. 2022, 11, 1013–1023. [Google Scholar] [CrossRef]
- Morachis-Valdez, G.; Dublán-García, O.; López-Martínez, L.X.; Galar-Martínez, M.; Saucedo-Vence, K.; Gómez-Oliván, L.M. Chronic exposure to pollutants in Madín Reservoir (Mexico) alters oxidative stress status and flesh quality in the common carp Cyprinus carpio. Environ. Sci. Pollut. Res. 2015, 22, 9159–9172. [Google Scholar] [CrossRef]
- Eckert, A.; Keil, U.; Kressmann, S.; Schindowski, K.; Leutner, S.; Leutz, S.; Muller, W.E. Effects of EGb 761 Ginkgo biloba extract on mitochondrial function and oxidative stress. Pharmacopsychiatry 2003, 36, s15–s23. [Google Scholar]
- Ibrahim, D.; Kishawy, A.T.Y.; Khater, S.I.; Khalifa, E.; Ismail, T.A.; Mohammed, H.A.; Elnahriry, S.S.; Tolba, H.A.; Sherief, W.; Farag, M.; et al. Interactive effects of dietary quercetin nanoparticles on growth, flesh antioxidant capacity and transcription of cytokines and Aeromonas hydrophila quorum sensing orchestrating genes in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2021, 119, 478–489. [Google Scholar] [CrossRef]
- Eisvand, F.; Razavi, B.M.; Hosseinzadeh, H. The effects of Ginkgo biloba on metabolic syndrome: A review. Phytother. Res. 2020, 34, 1798–1811. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Wan, X.L.; Zhang, X.H.; Zhao, L.G.; He, J.T.; Zhang, J.F.; Zhang, L.L.; Wang, T. Effect of supplemental fermented Ginkgo biloba leaves at different levels on growth performance, meat quality, and antioxidant status of breast and thigh muscles in broiler chickens. Poult. Sci. 2017, 96, 869–877. [Google Scholar] [CrossRef]
- Kulkeaw, K.; Sugiyama, D. Zebrafish erythropoiesis and the utility of fish as models of anemia. Stem Cell Res. Ther. 2012, 3, 55. [Google Scholar] [PubMed]
- Çimen, M.Y.B. Free radical metabolism in human erythrocytes. Clin. Chim. Acta 2008, 390, 1–11. [Google Scholar] [CrossRef]
- Clemens, M.R.; Ruess, M.; Bursa, Z.; Waller, H.D. The relationship between lipid composition of red blood cells and their susceptibility to lipid peroxidation. Free Radic. Res. Commun. 1987, 3, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.P.; Hultin, H.O. Contributions of Blood and Blood Components to Lipid Oxidation in Fish Muscle. J. Agric. Food Chem. 2002, 50, 555–564. [Google Scholar] [CrossRef]
- Köse, K.; Dogan, P. Lipoperoxidation Induced by Hydrogen Peroxide in Human Erythrocyte Membranes. J. Int. Med. Res. 1995, 23, 9–18. [Google Scholar] [CrossRef]
- Sinha, A.K.; Zinta, G.; AbdElgawad, H.; Asard, H.; Blust, R.; De Boeck, G. High environmental ammonia elicits differential oxidative stress and antioxidant responses in five different organs of a model estuarine teleost (Dicentrarchus labrax). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 174–175, 21–31. [Google Scholar] [CrossRef]
- Zheng, T.; Jia, R.; Cao, L.; Du, J.; Gu, Z.; He, Q.; Xu, P.; Yin, G. Alleviative effects of Ginkgo biloba extract on oxidative stress, inflammatory response and immune suppression induced by long-term glyphosate exposure in tilapia (Oreochromis niloticus). Aquaculture 2022, 546, 737325. [Google Scholar] [CrossRef]






| Ingredients | % | Proximate Analysis 3 | % |
|---|---|---|---|
| Fish meal | 27.00 | Dry matter | 92.46 |
| Soybean meal | 36.00 | Crude protein | 34.30 |
| Wheat flour | 32.50 | Crude lipid | 6.25 |
| Ca(H2PO4)2 | 0.50 | Crude Ash | 5.78 |
| Corn oil | 2.00 | ||
| Vitamin mixture 1 | 1.00 | ||
| Mineral mixture 2 | 1.00 |
| Extracts | Flavonoids (mg/g Dry Extracts) | T-AOC (mM of Trolox) | MCA (% of Control) |
|---|---|---|---|
| PEE | 11.49 ± 0.64 a | 0.45 ± 0.03 a | 16.20 ± 0.93 a |
| EAE | 70.60 ± 5.05 d | 1.02 ± 0.05 d | 70.42 ± 2.44 d |
| EE | 32.79 ± 2.29 c | 0.88 ± 0.06 c | 40.38 ± 2.34 c |
| AQE | 19.94 ± 1.10 b | 0.69 ± 0.03 b | 24.30 ± 1.53 b |
| Treatment | PO (% of Control) | CD (% of Control) | MDA (% of Control) |
|---|---|---|---|
| PEE | 72.38 ± 4.50 d | 67.69 ± 3.23 c | 69.09 ± 5.18 c |
| EAE | 39.90 ± 2.43 a | 40.79 ± 3.07 a | 44.85 ± 2.42 a |
| EE | 55.84 ± 2.39 b | 54.97 ± 2.49 b | 58.99 ± 2.52 b |
| AQE | 64.23 ± 2.22 c | 65.20 ± 2.49 c | 69.29 ± 2.99 c |
| Treatment | PO (% of Control) | CD (% of Control) | MDA (% of Control) |
|---|---|---|---|
| PEE | 59.35 ± 2.44 d | 62.22 ± 3.13 c | 55.10 ± 2.63 c |
| EAE | 33.97 ± 1.99 a | 37.50 ± 2.08 a | 34.61 ± 1.88 a |
| EE | 39.06 ± 2.80 b | 50.22 ± 2.52 b | 45.54 ± 2.62 b |
| AQE | 49.18 ± 1.67 c | 53.00 ± 2.53 b | 54.90 ± 2.04 c |
| Retention Time (min) | Compound Name | Molecular Weight (amu) | Molecular Formula | Matching Degree (%) |
|---|---|---|---|---|
| 4.838 | 1,5,6,7-Tetramethylbicyclo[3.2.0]hept-6-en-3-one | 164 | C11H16O | 80 |
| 7.472 | 1,4-Cyclohexadiene, 3,3,6,6-tetramethyl- | 136 | C10H16 | 80 |
| 1,5,6,7-Tetramethylbicyclo[3.2.0]hept-6-en-3-one | 164 | C11H16O | 76 | |
| 7.777 | Phytol, acetate | 338 | C22H42O2 | 82 |
| 1,2-Dihexylcyclopropene | 208 | C15H28 | 78 |
| EAE (g kg−1 Diet) | 0.0 | 1.0 | 2.0 | 3.0 | 4.0 | 5.0 | 6.0 |
|---|---|---|---|---|---|---|---|
| IBW (g fish−1) | 14.78 ± 0.37 a | 14.75 ± 0.36 a | 14.80 ± 0.40 a | 14.79 ± 0.29 a | 14.81 ± 0.38 a | 14.69 ± 0.34 a | 14.57 ± 0.40 a |
| FBW (g fish−1) | 56.06 ± 2.18 b | 59.14 ± 2.32 cd | 61.61 ± 2.09 e | 62.57 ± 2.33 e | 60.92 ± 2.07 de | 57.93 ± 2.46 bc | 52.92 ± 2.07 a |
| WG (g fish−1) | 41.27 ± 2.16 b | 44.38 ± 1.39 cd | 46.82 ± 1.15 e | 47.78 ± 1.46 e | 46.14 ± 1.14 de | 43.19 ± 1.60 bc | 38.35 ± 1.74 a |
| SGR (% d−1) | 2.22 ± 0.07 b | 2.31 ± 0.03 cd | 2.38 ± 0.03 de | 2.40 ± 0.03 e | 2.36 ± 0.03 de | 2.28 ± 0.04 bc | 2.15 ± 0.08 a |
| FI (g fish−1) | 67.38 ± 2.83 b | 71.15 ± 2.96 bc | 72.40 ± 1.99 c | 73.82 ± 2.32 c | 71.87 ± 2.45 c | 67.83 ± 2.55 b | 62.88 ± 2.54 a |
| FE (%) | 61.28 ± 2.61 a | 62.47 ± 3.08 a | 64.72 ± 2.55 a | 64.80 ± 3.31 a | 64.23 ± 1.99 a | 63.78 ± 3.83 a | 61.08 ± 3.81 a |
| SR (%) | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| EAE (g kg−1 Diet) | 0.0 | 1.0 | 2.0 | 3.0 | 4.0 | 5.0 | 6.0 |
|---|---|---|---|---|---|---|---|
| Trypsin (U mg−1 protein) | 769.22 ± 159.11 b | 1645.93 ± 213.49 d | 1694.70 ± 108.87 d | 1253.24 ± 113.93 c | 813.20 ± 167.95 b | 668.35 ± 120.94 ab | 487.08 ± 93.94 a |
| Lipase (U g−1 protein) | 35.80 ± 7.16 a | 36.36 ± 3.94 a | 38.12 ± 4.13 a | 53.00 ± 8.35 b | 63.15 ± 7.02 bc | 66.61 ± 10.53 c | 62.35 ± 6.93 bc |
| Amylase (U mg−1 protein) | 1.00 ± 0.09 a | 1.04 ± 0.05 ab | 1.14 ± 0.14 abc | 1.27 ± 0.09 bc | 1.32 ± 0.13 c | 1.20 ± 0.08 abc | 1.11 ± 0.08 abc |
| ASA (U g−1 protein) | 61.86 ± 2.93 ab | 61.37 ± 3.44 ab | 64.83 ± 1.07 bc | 65.19 ± 1.75 bc | 67.37 ± 4.62 c | 63.28 ± 0.37 b | 58.87 ± 0.75 a |
| AHR (U mg−1 protein) | 147.72 ± 23.70 a | 150.65 ± 7.34 ab | 151.98 ± 9.38 ab | 193.46 ± 8.69 c | 179.62 ± 18.40 bc | 180.38 ± 22.10 bc | 159.08 ± 10.15 ab |
| SOD (U mg−1 protein) | 83.27 ± 2.71 ab | 83.35 ± 5.23 ab | 90.66 ± 3.09 bc | 95.95 ± 4.44 c | 90.12 ± 2.86 bc | 80.63 ± 9.06 a | 78.95 ± 3.03 a |
| CAT (U mg−1 protein) | 20.40 ± 3.66 a | 22.99 ± 4.17 ab | 25.47 ± 3.48 abc | 31.19 ± 2.13 cd | 38.34 ± 3.47 e | 35.77 ± 4.99 de | 26.93 ± 4.68 bc |
| GPx (U mg−1 protein) | 278.08 ± 17.38 a | 299.74 ± 34.13 ab | 311.95 ± 36.06 ab | 335.49 ± 49.67 ab | 341.01 ± 26.82 b | 297.89 ± 26.77 ab | 274.40 ± 18.00 a |
| GST (U mg−1 protein) | 87.35 ± 8.44 a | 86.43 ± 7.52 a | 93.24 ± 6.71 a | 95.06 ± 4.03 a | 91.25 ± 9.55 a | 91.41 ± 12.90 a | 92.37 ± 2.51 a |
| MDA (nmol mg−1 protein) | 12.21 ± 2.31 d | 9.81 ± 1.84 c | 5.90 ± 0.59 b | 4.41 ± 0.54 ab | 3.79 ± 0.28 a | 4.65 ± 0.56 ab | 4.94 ± 0.76 ab |
| H2O2 (mmol g−1 protein) | 31.52 ± 0.55 b | 29.43 ± 2.35 ab | 29.53 ± 1.28 ab | 29.29 ± 1.41 ab | 28.27 ± 0.04 a | 27.76 ± 0.04 a | 27.91 ± 0.04 a |
| GSH (mg g−1 protein) | 7.16 ± 1.24 a | 8.53 ± 1.16 ab | 8.87 ± 0.82 bc | 11.77 ± 1.07 d | 10.00 ± 0.66 c | 7.76 ± 0.79 ab | 7.38 ± 0.29 a |
| EAE (g kg−1 Diet) | 0.0 | 1.0 | 2.0 | 3.0 | 4.0 | 5.0 | 6.0 |
|---|---|---|---|---|---|---|---|
| Trypsin (U mg−1 protein) | 1092.62 ± 33.17 ab | 1131.91 ± 75.60 b | 1158.46 ± 97.24 b | 1131.90 ± 42.17 b | 1133.46 ± 48.90 b | 1145.19 ± 37.27 b | 1007.35 ± 61.41 a |
| Amylase (U mg−1 protein) | 0.80 ± 0.10 a | 1.23 ± 0.05 b | 1.22 ± 0.19 b | 1.16 ± 0.03 b | 0.86 ± 0.08 a | 0.87 ± 0.04 a | 0.84 ± 0.06 a |
| Na+/K+-ATPase (U mg−1 protein) | 3.70 ± 0.39 b | 3.87 ± 0.13 b | 6.75 ± 0.77 d | 4.89 ± 0.80 c | 4.66 ± 0.65 c | 3.40 ± 0.26 b | 2.48 ± 0.19 a |
| AKP (U mg−1 protein) | 280.87 ± 22.65 a | 294.03 ± 5.11 ab | 302.80 ± 9.95 ab | 306.46 ± 12.65 b | 290.77 ± 11.13 ab | 294.11 ± 6.50 ab | 280.37 ± 25.01 a |
| ASA (U g−1 protein) | 46.65 ± 3.41 a | 49.27 ± 1.92 ab | 47.89 ± 2.51 ab | 51.57 ± 1.88 b | 51.45 ± 2.68 b | 46.39 ± 3.81 a | 46.52 ± 2.12 a |
| GST (U mg−1 protein) | 75.74 ± 8.43 a | 113.66 ± 16.32 b | 141.82 ± 26.15 c | 155.27 ± 24.03 c | 105.04 ± 10.99 b | 103.62 ± 16.26 b | 67.26 ± 6.78 a |
| SOD (U mg−1 protein) | 74.64 ± 15.90 a | 135.32 ± 15.79 c | 151.70 ± 5.24 c | 148.36 ± 12.97 c | 97.37 ± 13.14 b | 96.45 ± 15.99 b | 83.75 ± 6.83 b |
| CAT (U mg−1 protein) | 13.62 ± 1.32 a | 16.27 ± 3.37 ab | 18.58 ± 1.60 bc | 18.64 ± 1.52 bc | 18.44 ± 1.59 bc | 19.14 ± 2.52 bc | 23.16 ± 4.21 c |
| GPx (U mg−1 protein) | 421.62 ± 59.02 b | 422.56 ± 56.11 b | 419.92 ± 67.01 b | 418.34 ± 65.99 b | 597.12 ± 6.12 c | 332.33 ± 37.49 ab | 258.80 ± 9.29 a |
| GSH (mg g−1 protein) | 3.06 ± 0.29 a | 3.06 ± 0.25 a | 3.42 ± 0.11 a | 3.45 ± 0.20 a | 4.25 ± 0.57 a | 8.86 ± 0.80 b | 12.15 ± 1.75 c |
| H2O2 (mmol g−1 protein) | 17.51 ± 1.70 c | 12.97 ± 1.48 ab | 11.91 ± 2.29 ab | 11.12 ± 1.79 a | 10.01 ± 2.15 a | 12.68 ± 1.98 ab | 14.54 ± 2.09 b |
| EAE (g kg−1 Diet) | 0.0 | 1.0 | 2.0 | 3.0 | 4.0 | 5.0 | 6.0 |
|---|---|---|---|---|---|---|---|
| ASA (U g−1 protein) | 53.53 ± 8.94 ab | 56.23 ± 12.07 ab | 57.46 ± 13.83 ab | 67.29 ± 3.93 bc | 74.73 ± 9.17 c | 61.39 ± 7.02 abc | 51.05 ± 5.46 a |
| MDA (nmol mg−1 protein) | 6.48 ± 0.48 c | 6.19 ± 0.63 bc | 5.30 ± 0.73 ab | 4.40 ± 0.72 a | 4.95 ± 0.68 a | 6.89 ± 0.22 c | 9.65 ± 1.04 d |
| SOD (U mg−1 protein) | 18.27 ± 2.06 ab | 19.26 ± 1.40 abc | 21.15 ± 2.09 bc | 21.57 ± 1.59 c | 18.63 ± 1.76 ab | 18.72 ± 1.80 a | 17.76 ± 0.83 a |
| CAT (U mg−1 protein) | 5.30 ± 0.69 a | 5.23 ± 0.73 a | 5.38 ± 0.78 a | 6.26 ± 1.34 ab | 9.49 ± 2.07 c | 8.01 ± 1.93 bc | 5.56 ± 0.94 a |
| GPx (U mg−1 protein) | 64.17 ± 9.60 a | 69.38 ± 11.51 a | 77.02 ± 9.11 ab | 117.36 ± 19.23 d | 107.71 ± 7.37 cd | 98.10 ± 9.13 bcd | 92.48 ± 13.13 bc |
| GSH (mg g−1 protein) | 6.86 ± 0.72 a | 8.48 ± 0.56 b | 11.40 ± 1.66 c | 8.31 ± 0.54 ab | 7.52 ± 0.61 ab | 7.53 ± 0.96 ab | 7.70 ± 0.88 ab |
| GST (U mg−1 protein) | 25.49 ± 1.40 a | 35.84 ± 4.63 a | 48.72 ± 7.91 b | 50.47 ± 8.46 b | 48.15 ± 8.25 b | 62.09 ± 9.21 c | 67.43 ± 9.18 c |
| EAE (g kg−1 Diet) | 0.0 | 1.0 | 2.0 | 3.0 | 4.0 | 5.0 | 6.0 |
|---|---|---|---|---|---|---|---|
| PAC (μmol mL−1) | 352.4 ± 25.46 b | 348.46 ± 33.09 ab | 338.56 ± 27.31 ab | 331.5 ± 23.4 ab | 330.13 ± 22.56 ab | 333.73 ± 16.93 ab | 310.25 ± 14.57 a |
| GOT (U L−1) | 121.8 ± 18.41 b | 76.2 ± 14.42 a | 85.0 ± 9.07 a | 88.9 ± 15.10 a | 115.3 ± 6.88 b | 115.4 ± 9.78 b | 124.9 ± 19.68 b |
| GPT (U L−1) | 159.80 ± 27.02 d | 120.83 ± 18.28 c | 62.36 ± 8.47 ab | 34.63 ± 3.37 a | 95.37 ± 1.33 bc | 227.92 ± 9.46 e | 237.34 ± 36.36 e |
| EAE (g kg−1 Diet) | 0.0 | 1.0 | 2.0 | 3.0 | 4.0 | 5.0 | 6.0 |
|---|---|---|---|---|---|---|---|
| Na+/K+-ATPase (U mg−1 Hb) | 1.61 ± 0.02 ab | 1.63 ± 0.09 ab | 1.67 ± 0.1 b | 1.54 ± 0.17 ab | 1.52 ± 0.08 ab | 1.53 ± 0.05 ab | 1.48 ± 0.12 a |
| GPT (U g−1 Hb) | 5.24 ± 0.29 a | 5.82 ± 0.64 ab | 6.59 ± 0.79 b | 6.63 ± 0.55 b | 6.77 ± 0.6 bc | 7.68 ± 0.89 c | 5.75 ± 0.55 ab |
| LDH (U g−1 Hb) | 571.43 ± 33.46 d | 565.58 ± 21.61 d | 192.88 ± 30.55 a | 259.4 ± 32.33 b | 269.48 ± 46.38 b | 281.31 ± 64.67 b | 378.16 ± 39.09 c |
| Met-Hb (g L−1) | 1.84 ± 0.03 d | 1.81 ± 0.05 d | 1.79 ± 0.04 cd | 1.64 ± 0.08 b | 1.42 ± 0.08 a | 1.66 ± 0.14 bc | 1.77 ± 0.13 bcd |
| O2•− (U g−1 Hb) | 42.3 ± 2.97 bc | 41.42 ± 2.94 bc | 39.53 ± 1.20 ab | 38.76 ± 3.76 ab | 35.61 ± 2.52 a | 40.14 ± 2.34 ab | 45.06 ± 3.99 c |
| H2O2 (mmol g−1 Hb) | 35.03 ± 3.83 bc | 34.46 ± 3.53 bc | 31.28 ± 1.27 b | 26.39 ± 0.69 a | 36.64 ± 3.02 bc | 39.60 ± 2.91 c | 46.06 ± 2.88 d |
| MDA (nmol mg−1 Hb) | 4.82 ± 0.44 c | 3.50 ± 0.51 b | 1.46 ± 0.14 a | 1.94 ± 0.31 a | 3.94 ± 0.36 b | 3.85 ± 0.72 b | 4.68 ± 0.40 c |
| SOD (U mg−1 Hb) | 89.68 ± 12.27 a | 101.21 ± 3.72 ab | 130.25 ± 13.88 d | 131.87 ± 9.72 d | 121.88 ± 5.47 cd | 114.50 ± 16.77 bcd | 104.73 ± 18.35 abc |
| CAT (U mg−1 Hb) | 6.55 ± 0.98 a | 6.93 ± 1.13 ab | 6.85 ± 1.02 ab | 7.11 ± 0.86 ab | 8.18 ± 0.90 b | 7.58 ± 0.89 ab | 7.69 ± 0.92 ab |
| GSH (mg g−1 Hb) | 7.41 ± 0.35 a | 7.44 ± 0.23 a | 8.25 ± 1.14 a | 8.12 ± 0.79 a | 9.44 ± 0.71 b | 9.62 ± 0.55 b | 15.35 ± 1.06 c |
| GR (U g−1 Hb) | 33.89 ± 2.39 a | 37.24 ± 1.79 b | 42.98 ± 2.91 c | 45.14 ± 2.36 cd | 46.44 ± 1.49 cd | 46.05 ± 2.02 d | 48.25 ± 1.53 d |
| EAE (g kg−1 Diet) | 0.0 | 1.0 | 2.0 | 3.0 | 4.0 | 5.0 | 6.0 |
|---|---|---|---|---|---|---|---|
| H2O2 (mmol g−1 protein) | 28.47 ± 3.59 c | 29.29 ± 4.30 c | 15.46 ± 3.19 b | 13.12 ± 1.36 ab | 11.35 ± 0.42 a | 10.2 ± 0.80 a | 9.79 ± 0.29 a |
| AHR (U mg−1 protein) | 173.24 ± 23.39 a | 177.68 ± 9.20 a | 175.41 ± 8.73 a | 180.41 ± 2.98 ab | 211.97 ± 13.18 c | 201.27 ± 7.67 bc | 195.15 ± 12.74 abc |
| MDA (nmol mg−1 protein) | 9.06 ± 1.79 c | 5.49 ± 0.44 b | 5.13 ± 0.53 ab | 3.90 ± 0.58 a | 5.10 ± 0.32 ab | 4.84 ± 0.54 ab | 6.15 ± 0.70 b |
| CAT (U mg−1 protein) | 10.47 ± 0.71 a | 14.27 ± 2.63 b | 14.19 ± 1.87 b | 15.98 ± 0.58 b | 16.52 ± 0.80 b | 16.47 ± 1.58 b | 23.83 ± 2.83 c |
| GSH (mg g−1 protein) | 6.93 ± 0.70 a | 11.31 ± 1.40 b | 14.03 ± 0.98 bcd | 15.76 ± 2.33 cd | 16.48 ± 3.12 d | 13.32 ± 0.24 bc | 6.06 ± 0.54 a |
| GST (U mg−1 protein) | 66.02 ± 12.48 a | 102.49 ± 7.35 b | 140.54 ± 28.63 c | 185.63 ± 36.47 d | 174.72 ± 10.52 d | 133.81 ± 24.64 bc | 109.24 ± 17.77 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Xu, J.; Wu, M.; Li, H.; Yang, Q.; Feng, L. Extract of Ginkgo biloba Leaves (EGb) Decrease Lipid Oxidation in Fish Feed and Meat and Enhance Growth and Antioxidant Capacity in Jian Carp (Cyprinus carpio var. Jian). Fishes 2023, 8, 564. https://doi.org/10.3390/fishes8110564
Chen G, Xu J, Wu M, Li H, Yang Q, Feng L. Extract of Ginkgo biloba Leaves (EGb) Decrease Lipid Oxidation in Fish Feed and Meat and Enhance Growth and Antioxidant Capacity in Jian Carp (Cyprinus carpio var. Jian). Fishes. 2023; 8(11):564. https://doi.org/10.3390/fishes8110564
Chicago/Turabian StyleChen, Gangfu, Jing Xu, Min Wu, Huatao Li, Qihui Yang, and Lin Feng. 2023. "Extract of Ginkgo biloba Leaves (EGb) Decrease Lipid Oxidation in Fish Feed and Meat and Enhance Growth and Antioxidant Capacity in Jian Carp (Cyprinus carpio var. Jian)" Fishes 8, no. 11: 564. https://doi.org/10.3390/fishes8110564
APA StyleChen, G., Xu, J., Wu, M., Li, H., Yang, Q., & Feng, L. (2023). Extract of Ginkgo biloba Leaves (EGb) Decrease Lipid Oxidation in Fish Feed and Meat and Enhance Growth and Antioxidant Capacity in Jian Carp (Cyprinus carpio var. Jian). Fishes, 8(11), 564. https://doi.org/10.3390/fishes8110564