1. Introduction
Nutrition is the critical aspect that influences the productivity and profitability of the aquaculture industry. Commercial diets are formulated with the most available and cost-effective raw materials, considering their nutritional value, nutrient balance and bioavailability. The main challenge in feed formulation is fulfilling the gap between the nutrient requirement of animals and the available nutrient content in the feed. Fortification or enrichment of the diet with specific individual nutrients or blends of nutrients can fulfill the nutrient deficits while improving the growth and health performance of farm animals [
1]. Moreover, when raised in high-density culture conditions, fish are highly susceptible to infectious diseases, which can cause significant economic losses for the farm. Strengthening fish immunity can reduce the risk of emerging and spreading sudden disease outbreaks during immune-deprived conditions. Dietary immunostimulants supplementation is beneficial in improving fish immune responses by enhancing the activity of immune cells such as phagocytes and lymphocytes and the production of cytokines and antibodies [
2]. Naturally originated substances such as plant extracts, prebiotics and probiotics have been shown to convey promising outcomes as immunostimulants [
3,
4]. Numerous studies have shown that specific nutrients, including amino acids, fatty acids, nucleotides, vitamins and minerals, significantly boost animal immune functions [
5,
6].
Nucleotides are a conditionally essential nutrient in diets for fishes since they are vital in energy metabolism, DNA and RNA synthesis, encoding genetic information, physiological mediators in signal transduction and being a component of coenzymes, cellular agonists and allosteric effectors [
7,
8,
9]. Nucleotides are synthesized in the cell using amino acids, folic acid and CO
2, but it is more efficient to use externally supplied nucleotides [
10]. Previous studies showed that dietary supplementation of nucleotides improved the feed acceptance, growth performance, intestinal structure and immune responses in numerous fish species, including olive flounder,
Paralichthys olivaceus [
11], Nile tilapia,
Oreochromis niloticus [
12], Atlantic salmon,
Salmo salar [
13] and common carp,
Cyprinus carpio [
14]. Burrells et al. [
15] showed that the inclusion of nucleotides with β-glucan in the diet for rainbow trout,
Oncorhynchus mykiss, reduced the cumulative mortality by approximately 18% during the bacterial challenge against
Vibrio anguillarum. Therefore, exogenous nucleotide supplementation is essential when aquatic animals are in fast-growing stages or under tissue injuries and stress conditions [
16].
Vitamins and provitamin sources have been studied for fish species since the early twentieth century, and they are required in trace amounts to maintain the growth and health of animals [
17,
18,
19]. Vitamin C (L-ascorbic acid) is an essential micronutrient required for antioxidant activities, immune competence and disease resistance, stress tolerance, steroidogenesis in ovarian follicle cells, connective tissue formation, wound healing and normal physiological functions in fish [
9,
20]. However, external vitamin C sources are essential for teleost fish since they are unable to
de novo synthesize owing to the lack of L-gulonolactone oxidase in the vitamin C biosynthesis pathway [
21]. Insufficient levels of dietary vitamin C were reported to cause deficiency symptoms in fish, such as poor growth, reduced immunity, skin hemorrhage, exophthalmia, anorexia, anemia, scoliosis, lordosis, shortened operculum and increased mortality [
22,
23]. In addition, vitamins C and E are hydrophilic and lipophilic antioxidants that scavenge ROS in aqueous and lipidic environments, respectively [
19]. Vitamin E, α-tocopherol, is an important cell membrane component in preventing the oxidation of polyunsaturated membrane phospholipids [
24]. Through the antioxidant activity of vitamin E, vitamin C reduces tocopheroxyl radical back to α-tocopherol, which is known as the synergistic effect between vitamins C and E [
9,
25]. Lee et al. [
26] observed significantly increased weight gain (WG), health improvement and reduced liver mercury deposition with dietary selenomethionine, vitamins C and E. Vitamin E is an essential nutrient for cell membrane formation, maintaining the functional and structural integrity of cells, growth, immunity, reproduction and preventing muscle degeneration [
24,
27]. Vitamin E deficiency in fish can cause white muscle fiber necrosis, anemia, depigmentation, erythropoiesis and ceroid pigmentation in the liver [
9].
Glucans are a group of polysaccharides derived from the cell wall materials of plants, fungi, algae and bacteria [
28]. Furthermore, they are branched or unbranched polymers comprised of β (1,4) and/or β (1,3) linked glucose-monomer units [
29]. Rodrigues et al. [
30] reported that β-glucan administration through different routes, such as dietary supplementation, immersion in baths and injections, improved fish immune responses and stress tolerance. β-glucan triggers host immunity against several pathogen infections since it can act as a pathogen-associated molecular pattern (PAMP) [
31]. The interaction between PAMP and pattern recognition receptors of monomorphonuclear phagocytes and neutrophils of the host can activate phagocytosis, microbial killing, cytokinin production and innate immune memory [
32,
33]. Dietary supplementation of β-glucan is known to significantly alter the gut bacterial populations and reduce the pathogen infections of
Streptococcus iniae,
Aeromonas salmonicida and
A. hydrophila [
34,
35]. Previous studies have shown that β-glucan improved feed utilization and growth in red seabream (
Pagrus major) and Pacific white shrimp (
Litopenaeus vannamei) [
36,
37]. Especially, β-glucan plays a vital role in improving innate immunity, including lysozyme, ACH50, respiratory burst activity and immune- and cytokine production-related gene expressions in fishes [
35,
38,
39,
40].
Olive flounder is the most important mariculture species in the Republic of Korea, representing approximately 50% of the country’s production [
41]. However, due to disease outbreaks, a wide range of antibiotics has been used to control infectious diseases, leading to drug-resistant bacteria and remnants of antibiotics in fish products and water contamination [
42]. Dietary immunostimulants supplementation can be a promising strategy to minimize antibiotic usage in the industry. Further, new approaches are required to improve nutritional formulations that aim to simultaneously promote growth and health for the progress of the aquaculture industry. Although previous studies identified the effects of individual immunostimulants and their optimum level, limited studies have evaluated the combinational effects of the individual immunostimulant. ROVIMAX
® HB Ultra is a commercially available immunostimulant mixture containing nucleotides,
Saccharomyces cerevisiae derived β-glucan, vitamin C and vitamin E at optimal levels for olive flounder. Therefore, the objective of this study was to evaluate the effect of dietary supplementation of a functional immunostimulant mixture (FIM), including nucleotide, β-glucan and vitamins C and E, on growth performance, feed utilization, morphometric indices, intestinal histology, hematological parameters, digestive enzyme activity, innate immunity, antioxidant capacity and inflammation-related gene expressions of olive flounder.
2. Materials and Methods
2.1. Experimental Diets
The basal diet (control) was formulated with 62% sardine fish meal and 4.5% fish oil (
Table 1). Another three diets were formulated by incorporating 0.5, 1.0 and 1.5% of FIM (ROVIMAX
® HB Ultra; a mixture of 1.5% nucleotides, 15.5%
Saccharomyces cerevisiae derived β-glucan, 10.0% vitamin C and 2.5% vitamin E) into the basal diet at the expense of wheat flour (designated as HB0.5, HB1.0 and HB1.5, respectively). All the ingredients were measured according to the formulations and thoroughly mixed after adding fish oil and 10% distilled water. Then, the dough was pelleted using a pellet machine (SP-50, Kumkang Engineering, Daegu, Republic of Korea) and air-dried at 25 °C for 8 h in an electric drier (SI-2400, Shinil General Drier Co., Ltd., Daegu, Republic of Korea). Diets were kept at −20 °C until they were used. Moisture and ash contents of the diet were determined according to AOAC [
43]. Crude protein was analyzed by a Kjeltec Analyzer Unit 2300 (FOSS, Hillerød, Denmark) and crude lipid was determined according to Folch et al. [
44]. The experimental diets were isonitrogenous and isolipidic (56.38% crude protein and 11.70% crude lipid). The amino acid levels were analyzed by reversed-phase high-performance liquid chromatography (HPLC) according to the method described by Henderson et al. [
45]. Amino acid compositions of the experimental diets are presented in
Table 2. Briefly, diet samples were hydrolyzed with 6 N hydrochloric acid at 112 °C for 24 h and derivatized. Then, separation was performed using the Waters HPLC system (binary pump model 1525, autosampler model 717 Plus and UV/Vis absorbance detector model 2489, California, CA, USA), and amino acid levels were quantified by analyzing chromatography peaks and compared to known amino acid standards. Vitamins C and E levels of diets were analyzed by the HPLC method at Pacific Lab Service (5 Woodlands Terrace, Singapore, 738430), and the levels for control, HB0.5, HB1.0 and HB1.5 diets were 0.24, 0.40, 0.80 and 1.27 g/kg, respectively, and 0.11, 0.20, 0.30 and 0.41 g/kg, respectively.
2.2. Fish and Feeding Trial
The feeding trial was conducted at the Marine Science Institute of Jeju National University (Jeju, Republic of Korea). The authors followed all applicable international, national and institutional guidelines for the care and use of animals (approval no: 2022-0028). Juvenile olive flounder were purchased from a private hatchery and transported to the institute. Experimental fish were fed a commercial diet (Suhyup feed, Republic of Korea; crude protein, 55%; crude lipid, 12%) and acclimated to the experimental conditions and facilities for two weeks. Six hundred fish (initial mean body weight, 26.3 ± 0.1 g) were randomly stocked in 20 cylindrical polyethylene tanks (240 L), each with 30 fish. Five replicate groups (tanks) of fish were allocated to each dietary treatment and tanks were randomly assigned. All the tanks were supplied with continuously filtered seawater at a flow rate of 3 L/min in a flow-through tank system and continuously aerated with an air stone. Water temperature, pH, salinity, ammonia and dissolved oxygen levels were 21.4 ± 2.4 ºC, 8.09 ± 0.2, 31 ± 1 ppt, 0.063 ± 0.007 mg/L and 9.67±0.61 ppm, respectively. In the fourth week of the feeding trial, fish were moved to 300 L tanks under the same conditions. Fish were hand-fed one of the experimental diets twice daily (08:00 and 17:00 h) until they were apparently satiated (3–5% body weight) for 12 weeks. Uneaten feeds were collected 20 min after each feeding, air-dried and weighed to assess feed intake (FI). The total biomass of each tank was measured every four weeks and the feeding was terminated 24 h before weighing.
2.3. Sample Collection and Analysis
After the 12-week feeding trial, the number of total fish in each tank was counted. Then, fish were individually weighed and length was measured to determine the growth performance, feed utilization, morphometric parameters and survivals. Before sampling, fourteen fish were randomly selected from each tank and anesthetized with 200 mg/L 2-phenoxyethanol (77699, Sigma-Aldrich., St. Louis, MO, USA). Blood samples were drawn from three fish, allowed to clot at room temperature for 30 min, centrifuged at 5000× g for 10 min at 4 °C in a high-speed refrigerated micro-centrifuge (Micro 17 TR: HanilBio Med Inc., Gwangju, Republic of Korea) and separated serum was stored at −80 °C. Another three blood samples were collected from three fish with heparinized syringes, centrifuged (5000× g, 30 min, 4 °C) to separate plasma samples and stored at −80 °C for further analysis. Five fish from each tank were kept at −20 °C for whole-body proximate composition analysis. Following blood sampling, stomach and intestine samples were collected from five remaining fish for digestive enzyme activity assay and samples were stored at −20 °C. Three fish were dissected under sterile conditions to collect liver samples for gene expression analysis and samples were frozen immediately in liquid nitrogen and stored in a −80 °C refrigerator. Another two intestine samples were fixed in 10% formalin fixative for histological observations.
The oxidative radical generation in phagocytes during oxidative burst was measured by the nitro-blue tetrazolium (NBT) assay following Anderson and Siwicki [
46] method and myeloperoxidase (MPO) activity was evaluated following the method proposed by Quade and Roth [
47]. Superoxide dismutase (SOD), catalase and glutathione peroxidase (GPx) activities were assayed using colorimetric test kits (Biovision Inc., California, CA, USA) following the instructions supplied by the manufacturer. Plasma total immunoglobulin level and lysozyme activity were determined following Siwicki et al. [
48] and Sankaran and Gurnani [
49] methods, respectively. Antiprotease activity was determined following Ellis [
50], with slight modifications suggested by Magnadóttir et al. [
51]. An automated blood analyzer (SLIM, SEAC Inc., Florence, Italy) was used to analyze blood biochemical parameters, including hemoglobin and plasma levels of glucose, total protein, triglyceride and cholesterol.
2.4. Intestinal Digestive Enzyme Analysis
For the digestive enzyme analysis, intestine samples were weighted and homogenized separately in distilled water (1 g of tissue in 1 mL of distilled water) using a homogenizer (Daihan Scientific Co, Ltd. Wonju, Republic of Korea). The homogenate was centrifuged at 10,000×
g for 15 min at 4 °C and the supernatant was collected. Then the crude enzyme extract was stored at −20 °C until used. The BioRad assay kit (Bio-Rad Laboratories, Inc., Seoul, Republic of Korea) was used to assess the total protein level of the supernatant and standard solutions were prepared using bovine serum [
52]. Worthington’s [
53] digestive enzyme analysis method was used to determine pepsin activity and 2% hemoglobin in 0.06 N HCl was reacted with crude enzyme extract as the substrate. The specific activity of pepsin was expressed as U = (Absorbance at 280 nm (supernatant)-Absorbance at 280 nm (blank)) × 1000/10 min × mg protein in the assay. Trypsin activity was measured according to Erlanger et al. [
54] and the used substrate was benzoyl-DL-arginine-p-nitroanilide (BAPNA, 911-77-3, Sigma-Aldrich, St. Louis, MO, USA). The absorbance at 410 nm was measured and trypsin amidase activity was presented as BAPNA unit/mg protein. Chymotrypsin activity was measured following the Erlanger et al. [
54] method with slight modifications proposed by Falcón-Hidalgo et al. [
55]. A total of 1 μM N-Succinyl-L-Ala-L-Ala-L-Pro-L-Phe-p-nitroanilide (SAPNA. 70967-97-4. Sigma-Aldrich, St. Louis, MO, USA) in 20 mM CaCl
2 and 50 mM Tris-HCl buffer (pH 8.0) were used as the substrate and buffer, respectively. Chymotrypsin activity was expressed as SAPNA units/mg protein. Lipase activity was determined following the Borlongan [
56] method, as explained in Medagoda et al. [
57] and values were expressed as 0.01 N NaOH volume required to neutralize the fatty acid released due to the hydrolysis of triglycerides in the stabilized standard olive oil emulsion (1.5 mL) by 1 mL of crude enzyme extract. Amylase activity was measured following the method proposed by Worthington [
53]. Briefly, 0.5 mL of enzyme extract was mixed with 0.5 mL of 1% starch solution in 20 nM sodium phosphate buffer (pH 6.9) containing 6.0 nM NaCl and the mixture was incubated at 37 °C for 3 min. The released maltose was reacted with 0.5 mL dinitro salicylic acid (609-99-4. Sigma-Aldrch, St. Louis, MO, USA) and incubated for 5 min in a boiling water bath. The absorbance was measured at 540 nm and the amount of maltose released during 3 min incubation was evaluated using a standard. Amylase activity was estimated as μmol maltose released/mg enzyme in reaction mixture × 3 min.
2.5. Quantitative Real-Time PCR (qRT-PCR) for Relative Gene Expression Analysis
Approximately 80 mg (50–100 mg) of liver tissue samples were used to extract total RNA. The tissue samples were homogenized in Trizol reagent (T9424. Sigma-Aldrich, St. Louis, MO, USA) and total RNA was isolated. RNA purity and concentration were measured at 260 nm using a NanoDrop spectrophotometer (Thermo Scientific, Delaware, SC, USA). For the complementary DNA synthesis, a 2.5 μg RNA was reverse transcribed using a Prime Script first-strand cDNA synthesis kit (Takara Bio Inc. Shiga, Japan). Quantitative real-time PCR (RT-qPCR) (Takara, Shiga, Japan) was used to analyze gene expressions and the 18S rRNA gene was used as the housekeeping gene. The primer sequence for the growth, innate immune and inflammation-related genes (insulin-like growth factor-1, IGF-1; insulin-like growth factor-binding protein, IGF-BP; toll-like receptor-3, TLR-3; Perforin; interleukin-6, IL-6; tumor necrosis factor-α, TNF-α; and transforming growth factor-β, TGF-β) were designed based on the published olive flounder cDNA sequences in the GenBank genetic sequence database (
Table 3). The real-time PCR program was performed one cycle at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s (denaturation), at 60 °C for 30 s (annealing), and at 72 °C for 20 s (extension). The relative expression ratios of immune-related genes were calculated according to Pfaffl [
58].
2.6. Histological Observations
The intestinal sections were dehydrated in graduated ethanol concentrations and embedded in paraffin. Then, the embedded intestine fractions were sectioned with a rotary microtome (Leica RM2235, Leica Biosystems, Richmond, IL USA) at 5 μm thickness and microscopic slides were prepared. Slides were stained with Alcian blue (AB, pH 2.5), periodic acid and Schiff reagents (PAS). Then, they were examined under a light microscope (Leica DM750. Leica microsystems, Heerbrugg, Switzerland) containing a digital camera (Leica ICC50 E. Leica microsystems, Heerbrugg, Switzerland). Villus heights were measured using an image analyzing software (×10, Leica Application Suite, version 4.13.0, Heerbrugg, Switzerland) and the mucus-secreting goblet cells were counted under the ×40 magnification of the microscope.
2.7. Statistical Analysis
All the experimental groups were subjected to a completely randomized design. The data were analyzed using one-way ANOVA in SPSS (version 24.0, International Business Machines Co., Armonk, NY, USA) statistical program. When ANOVA detected differences between groups, the differences in mean values were compared using Tukey’s HSD multiple comparison test (p < 0.05). Data were presented as mean ± standard deviation.
3. Results
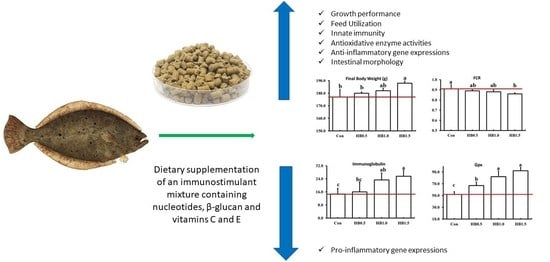
During the 12-week feeding trial, fish readily accepted all the experimental diets. Fish fed the HB1.5 diet showed significantly higher (
p < 0.05) final body weight (FBW), WG and specific growth rate (SGR) than fish fed the control and HB0.5 diets (
Table 4). Growth-related gene expressions of the fish were significantly upregulated (
p < 0.05) with dietary supplementation of FIM. Compared to the fish fed the control and HB0.5 diets, the relative expression of IGF-1 was significantly upregulated (
p < 0.05) in fish fed HB1.0 and HB1.5 diets. Moreover, significantly upregulated (
p < 0.05) IGF-BP gene expressions were observed in HB1.0 and HB1.5 groups than in the control group. Feed conversion ratio (FCR) was significantly lower (
p < 0.05) in the HB1.5 group compared to the control group, but other feed utilization parameters, FI, and protein efficiency ratio (PER) were not significantly affected (
p > 0.05) by FIM. Survival and morphometric parameters, including condition factor (CF), hepatosomatic index (HSI) and viscerosomatic index (VSI), showed no significant differences (
p > 0.05) among the dietary treatments.
Blood hemoglobin hematocrit and plasma total protein levels were significantly increased (
p < 0.05) in the fish fed the HB1.5 diet than in those fed the control diet (
Table 5). Blood glucose level was significantly reduced (
p < 0.05) in the HB1.5 group compared to the control and HB0.5 groups. Plasma triglyceride and cholesterol levels were not affected (
p > 0.05) by FIM. The dietary supplementation of the FIM significantly improved (
p < 0.05) almost all the immune parameters and antioxidant enzyme activities (
Table 6). Fish fed HB1.0 and HB1.5 showed significantly higher (
p < 0.05) lysozyme activity than the control diet. The total immunoglobulin level of the fish was significantly higher (
p < 0.05) in the HB1.5 group than in the control and HB0.5 groups. However, antiprotease activity showed no significant differences (
p > 0.05) among dietary groups. HB1.5 group showed a significantly higher (
p < 0.05) NBT activity than the control. MPO activity was significantly improved (
p < 0.05) in the fish of HB groups. SOD and catalase activities were significantly increased (
p < 0.05) with 1.5% FIM inclusion into the control diet. The fish fed HB1.0 and HB1.5 diets showed significantly higher (
p < 0.05) GPx activity than those fed the control and HB0.5 diets, and the HB0.5 group showed significantly increased GPx activity compared to the control group. Moreover, the dietary supplementation of the FIM at 1.5% in the control diet significantly upregulated (
p < 0.05) TLR-3 and perforin gene expressions.
The anti-inflammatory gene expression of TGF-β was significantly increased (
p < 0.05) with FIM supplementation at 1.5% (
Figure 1). Pro-inflammatory gene expressions of IL-6 and TNF-α were not significantly affected (
p > 0.05).
Digestive enzyme activities are presented in
Table 7. Amylase activity was significantly enhanced (
p < 0.05) at 1.0 or 1.5% of FIM inclusion levels. However, lipase, pepsin, trypsin and chymotrypsin activities were not significantly affected (
p > 0.05). Whole-body compositions of moisture, crude protein, crude lipid and ash were not significantly affected (
p > 0.05) with dietary FIM supplementation (
Table 8). Fish fed HB1.0 and HB1.5 diets showed significantly increased (
p < 0.05) villus height compared to fish fed the control and HB0.5 diets (
Table 9). The goblet cell counts were significantly higher (
p < 0.05) in fish fed HB1.0 and HB1.5 diets than those fed the control diet. Representative histology images of intestinal villus heights and goblet cell distribution are shown in
Figure 2 and
Figure 3.
4. Discussion
The dietary FIM for juvenile olive flounder showed increased growth, feed utilization and growth-related gene expressions. Chuchird et al. [
6] observed increased growth and feed efficiency in Pacific white shrimp fed the same FIM mixture-containing diet. Hossain et al. [
59] reported that exogenous nucleotide supplementation promoted the growth of red seabream and Borda et al. [
60] suggested that increased growth could be attributed to cell proliferation stimulating the ability of nucleotides. In our previous study, the inclusion of 0.1–0.4% inosine monophosphate in a fish meal-based diet enhanced the growth and health of olive flounder [
11]. Dietary nucleotide supplementation has shown enhanced growth in turbot (
Scophthalmus maximus), Nile tilapia, and rainbow trout [
61,
62,
63]. Metailler et al. [
61] reported that the growth performances of turbot larvae were significantly increased with 0.77% nucleotide supplementation in diet, assuming that this could be attributed to improved FI with increased diet palatability. Moreover, exogenous nucleotide supplementation can save metabolic energy costs, which could beneficially affect fish growth and health [
10]. However, Li et al. [
64] showed that the effect of dietary nucleotide supplementation on the growth of sub-adult or adult fish was limited compared to larval stages. Corresponding to the present study, Asaduzzaman et al. [
12] found that dietary supplementation of 0.2–0.4% inosine monophosphate significantly upregulated the IGF-1 and IGF-2 gene expressions in Nile tilapia. IGF-1 and IGF-BP genes play a vital role in cell growth by enhancing glucose and alanine uptake, myoblast proliferation and DNA, protein and glycogen synthesis [
65,
66]. Therefore, enhanced growth-related gene expressions with dietary FIM supplementation might have influenced the growth performance in this study. Vitamin C deficiency can lead to lordosis, scoliosis, shortened operculum and hyperplasia in the gill tissue affecting the growth performance. Vitamin C also plays an important role in the hydroxylation of lysine and proline for collagen biosynthesis, which is important in growth [
67]. Dietary FIM improved feed conversion, which might correlate with improved intestinal morphology. Previous studies demonstrated that dietary nucleotides and β-glucan increased villus heights and absorptive surface area, suppressed intestinal damage and promoted beneficial bifidobacterial colonization in many fishes, including Atlantic salmon [
13], European sea bass (
Dicentrarchus labrax L.) [
68], turbot [
69] and red seabream [
60]. Bueno et al. [
70] showed that exogenous nucleotide supplementation improved intestinal ultrastructure compared to natural nucleotide synthesis. Therefore, improved intestinal morphology of olive flounder may have beneficially affected the feed utilization and nutrient uptake. In an in vitro study, Jiang et al. [
71] observed that vitamin E significantly enhanced Na
+-K
+-ATPase activity in fish enterocytes. The Na
+-K
+-ATPase is involved in active nutrient transport in enterocytes, and increased activity can improve feed utilization.
The FIM supplementation in the diet significantly affected hematological parameters, including hemoglobin, hematocrit, total protein and glucose level of the fish. Cao et al. [
72] observed that dietary β-glucan incorporation significantly reduces the blood glucose level by downregulating the gene expression of sodium-glucose transporter-1 in the intestinal mucosa. Hematocrit and hemoglobin concentrations were significantly increased with the increasing FIM level. Tahmasebi-Kohyani et al. [
63] observed that dietary nucleotide supplementation increased hemoglobin, hematocrit and red and white blood cell counts. They suggested this may be due to an increased iron absorption with nucleotide supplementation. Further, they observed increased blood albumin and globulin levels with dietary nucleotide supplementation. Garcia et al. [
73] observed an erythrocyte-protecting effect through dietary vitamin C and E supplementations. Vitamin C and E contained in FIM might have increased the erythrocyte proliferation to obtain the above observations; thereby, it could promote efficient oxygen transportation in the body.
Lysozyme and antiprotease activities are vital in destroying bacteria by cell wall lysis and inhibiting bacterial proteases by trapping the enzymes or attaching them to their binding sites. Notably, these enzyme activities were increased with dietary FIM supplementation in this study. Carver et al. [
74] and Gil [
75] showed that dietary nucleotide supplementation influences macrophage and natural killer cell activation, enhancing lysozyme activity. Song et al. [
11] observed that 0.2–0.4% inosine monophosphate supplementation significantly improved the lysozyme activity and disease resistance of olive flounder against
S. iniae. Waagbø et al. [
76] found that the lysozyme activity of Atlantic salmon was increased with dietary vitamin C supplementation. Moreover, dietary nucleotide supplementation significantly affected immunoglobulin production by increasing lymphocyte production in fish [
77,
78]. Supporting the above findings, Leonardi et al. [
79] observed that supplementation of nucleotides in the Atlantic salmon diet showed significantly improved antibody production. An oral administration of β-glucan dramatically increased immunoglobulin in fish by binding to the β-glucan receptors of macrophages, leukocytes, neutrophils and NK cells [
80,
81]. Rodrigues et al. [
30] reported that the binding of β-glucan to relevant receptors acting as a PAMP stimulates all the immune activities, including phagocytosis and the production of interferon, cytokine and immunoglobulin. Therefore, the increased immunoglobulin content in the present study might be partially attributed to the β-glucan contained in the FIM mixture.
Respiratory burst activity, antioxidative activity and immune parameters were significantly improved with the graded levels of FIM supplementation. Nitroblue tetrazolium activity measures the intercellular superoxide concentration and the MPO enzyme converts hydrogen peroxide into hypochlorous acid in phagocytes during phagocytosis [
82,
83]. Dietary nucleotide supplementation improved phagocytic activities in fishes, including olive flounder and rainbow trout [
11,
75,
84]. Carver et al. [
74] reported that nucleotide supplementation in rats significantly increased the natural killer cell activation and macrophage activity. In a systematic review, Dawood and Kohio [
23] stated that vitamins C and E supplementation in the fish diet has beneficial effects on respiratory burst, phagocytic, lysozyme and complement activities. However, the underlying mechanism for increasing respiratory burst activity by dietary supplementation of nucleotides, β-glucan and vitamin C and E, or a combined effect, remains to be revealed.
In this study, the antioxidant activity in olive flounder was significantly increased. Similarly, Tie et al. [
85] observed upregulated Nrf2 gene expression, which is crucial in initiating the antioxidant enzyme gene expressions in grass carp,
Ctenopharyngodon Idella. They suggested that an increased nutrient availability with dietary nucleotide supplementation might have upregulated those gene expressions. Increased serum SOD, GPx and catalase activities were reported in red seabream, turbot and Nile tilapia when fed diets enriched with nucleotide over 0.15% [
59,
70,
86]. Supplementation of β-glucan in the diet also improved antioxidant enzyme activities in immune-suppressed tilapia exposed to atrazine herbicides [
87]. Caxico Vieira et al. [
88] suggested that the co-enzyme activity of vitamin C in carnitine and neurotransmitters synthesis could improve the antioxidant gene expressions in Nile tilapia with dietary vitamin C intake.
TLR is a PAMP-recognizing protein encoded by the TLR-3 gene, and it mediates cytokine production and activates inflammatory responses [
89]. Several studies have found that dietary supplementation of β-glucan or bacterial cell wall components (peptidoglycan and lipopolysaccharides) increases TLR-3 gene expression, emphasizing the importance of incorporating these compounds in fish feeds as a prophylactic method [
90,
91]. Therefore, dietary β-glucan supplementation might have significantly increased the TLR-3 gene expression in this study. Perforin is a cytosolic protein present in T-lymphocytes and natural killer cells, and it involves membrane pore-formation triggering calcium influx and initiating the cell death of targeted infected or damaged cells [
92]. Therefore, upregulation of perforin gene expression may be beneficial to renew the intestinal epithelium and protect olive flounder from pathogenic or parasitic infections.
According to
Table 7, digestive enzyme activities in the gut were increased with dietary FIM supplementation for olive flounder; even so, only the amylase activity showed a significant improvement. Hunt et al. [
93] observed significantly increased digestive enzyme activities in rainbow trout fed with a yeast-based nucleotide-incorporated diet, including pepsin, trypsin and lipase activities. They suggested that improved intestinal structure and increased surface area, and villi density might have increased digestive enzyme activities through dietary nucleotide supplementations. Bueno et al. [
70] showed that 0.25% dietary nucleotide supplementation significantly developed cellular ultrastructure and intestinal morphological characteristics in weaned rats. A similar mechanism might have led to enhancing the digestive enzyme activities in this study, but the exact influence of nucleotides, β-glucan and vitamin C and E supplementation on the digestive enzyme activity is yet to be revealed. Dietary β-glucan supplementation increases the gut digestive enzyme-secreting bacterial population by regulating the chyme viscosity [
94,
95]. However, information on the digestive enzyme production of bacteria in the intestine and their biological significance is limited.
Previous studies have shown that dietary β-glucan increases short-chain fatty acid assimilation by increasing bifidobacterial populations,
Lactobacilli and
Akkermansia spp. and alleviating colonization of detrimental bacterial species in the intestine. Furthermore, these beneficial bacteria species increase acetate, propionate and butyrate fatty acid production by the fermentation of non-digestible oligosaccharides, including β-glucans [
93,
96]. However, Medagoda and Lee [
97] showed body’s fatty acid profile correlates with the dietary fatty acid profile. Thus, the increased whole-body lipid content might have been due to the β-glucan contained in the FIM in the present study.
Nucleotide supplementation was reported to improve the intestinal structure, including increased villus height, lateral branching of the villus, mucosal height, gut wall thickness and enterocyte and microvillus height in fishes [
12,
69,
98,
99]. Bueno et al. [
70] revealed that nucleotides develop and recover the intestinal structure after the lactose-induced chronic diarrhea condition in rats suggesting that exogenous nucleotide supplementation spares the cost of metabolic energy in DNA and RNA synthesis in regenerating tissues. Hess and Greenberg [
100] reported that exogenous nucleotide supplementation is essential for intestinal tissue development since nucleotide demand is high in rapidly regenerating tissues. In addition, previous studies have shown that dietary nucleotide and β-glucan supplementation significantly increased the growth of gut health, improving beneficial microflora and stimulating mucus secretion from goblet cells [
60,
101,
102]. Rathore et al. [
103] observed improved normal cell structure, intestinal villi height and goblet cell count in Nile tilapia-fed vitamin C-containing diets. Vitamins C and E are antioxidant vitamins and reduce oxidative damage in the cell. Maintaining the structural integrity of the cell membrane by interacting between vitamins C and E is important to improve the absorption ability of the intestinal cells [
71]. Accordingly, the enrichment of nucleotide and vitamin C and E in the diet might have improved intestinal morphology.