Increased Temperature and Discharge Influence Overwinter Growth and Survival of Juvenile Salmonids in a Hydropeaking River: Simulating Effects of Climate Change Using Individual-Based Modelling
Abstract
:1. Introduction
2. Material and Methods
2.1. Model Description and Study Site
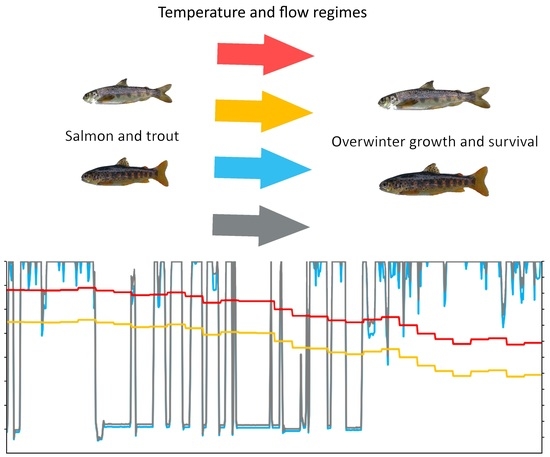
2.2. Flow and Temperature Scenarios
2.3. Data Analysis
3. Results
3.1. Specific Growth Rates
3.2. Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Vliet, M.T.; Franssen, W.H.; Yearsley, J.R.; Ludwig, F.; Haddeland, I.; Lettenmaier, D.P.; Kabat, P. Global river discharge and water temperature under climate change. Glob. Environ. Chang. 2013, 23, 450–464. [Google Scholar] [CrossRef]
- Isaak, D.J.; Wollrab, S.; Horan, D.; Chandler, G. Climate change effects on stream and river temperatures across the northwest US from 1980–2009 and implications for salmonid fishes. Clim. Chang. 2012, 113, 499–524. [Google Scholar] [CrossRef] [Green Version]
- O’Briain, R. Climate change and European rivers: An eco-hydromorphological perspective. Ecohydrology 2019, 12, e2099. [Google Scholar] [CrossRef]
- Huusko, A.R.I.; Greenberg, L.; Stickler, M.; Linnansaari, T.; Nykänen, M.; Vehanen, T.; Koljonen, S.; Louhi, P.; Alfredsen, K. Life in the ice lane: The winter ecology of stream salmonids. River Res. Appl. 2007, 23, 469–491. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. A review of the likely effects of climate change on anadromous Atlantic salmon Salmo salar and brown trout Salmo trutta, with particular reference to water temperature and flow. J. Fish Biol. 2009, 75, 2381–2447. [Google Scholar] [CrossRef]
- Heggenes, J.; Alfredsen, K.; Bustos, A.A.; Huusko, A.; Stickler, M. Be cool: A review of hydro-physical changes and fish responses in winter in hydropower-regulated northern streams. Environ. Biol. Fishes 2018, 101, 1–21. [Google Scholar] [CrossRef]
- Watz, J.; Aldvén, D.; Andreasson, P.; Aziz, K.; Blixt, M.; Calles, O.; Bjørnås, K.L.; Olsson, I.; Österling, M.; Stålhammar, S.; et al. Atlantic salmon in regulated rivers: Understanding river management through the ecosystem services lens. Fish Fish. 2022, 23, 478–491. [Google Scholar] [CrossRef]
- She, Y.; Hicks, F.; Andrishak, R. The role of hydro-peaking in freeze-up consolidation events on regulated rivers. Cold Reg. Sci. Technol. 2012, 73, 41–49. [Google Scholar] [CrossRef]
- Linnansaari, T.; Alfredsen, K.; Stickler, M.; Arnekleiv, J.V.; Harby, A.; Cunjak, R.A. Does ice matter? Site fidelity and movements by Atlantic salmon (Salmo salar L.) parr during winter in a substrate enhanced river reach. River Res. Appl. 2009, 25, 773–787. [Google Scholar] [CrossRef]
- Watz, J.; Bergman, E.; Piccolo, J.J.; Greenberg, L. Ice cover affects the growth of a stream-dwelling fish. Oecologia 2016, 181, 299–311. [Google Scholar] [CrossRef]
- Saltveit, S.J.; Halleraker, J.H.; Arnekleiv, J.V.; Harby, A. Field experiments on stranding in juvenile Atlantic salmon (Salmo salar) and brown trout (Salmo trutta) during rapid flow decreases caused by hydropeaking. Regul. Rivers Res. Manag. 2001, 17, 609–622. [Google Scholar] [CrossRef]
- Saltveit, S.J.; Bremnes, T.; Lindå, O.R. Effect of sudden increase in discharge in a large river on newly emerged Atlantic salmon (Salmo salar) and brown trout (Salmo trutta) fry. Ecol. Freshw. Fish 1995, 4, 168–174. [Google Scholar] [CrossRef]
- Jager, H.I.; DeAngelis, D.L. The confluences of ideas leading to, and the flow of ideas emerging from, individual-based modeling of riverine fishes. Ecol. Model. 2018, 384, 341–352. [Google Scholar] [CrossRef]
- Railsback, S.F.; Harvey, B.C.; Ayllón, D. Contingent trade-off decisions with feedbacks in cyclical environments: Testing alternative theories. Behav. Ecol. 2020, 31, 1192–1206. [Google Scholar] [CrossRef]
- Railsback, S.F.; Harvey, B.C.; Ayllón, D. Importance of the daily light cycle in population–habitat relations: A simulation study. Trans. Am. Fish. Soc. 2021, 150, 130–143. [Google Scholar] [CrossRef]
- Railsback, S.F.; Harvey, B.C.; Ayllón, D. InSTREAM 7.3 User Manual: Model Description, Software Guide, and Application Guide; USDA Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2022.
- Hajiesmaeili, M.; Addo, L.; Watz, J.; Railsback, S.F.; Piccolo, J.J. Individual-based modelling of hydropeaking effects on brown trout and Atlantic salmon in a regulated river. River Res. Appl. 2023, 39, 522–537. [Google Scholar] [CrossRef]
- Railsback, S.F.; Ayllón, D.; Harvey, B.C. InSTREAM 7: Instream flow assessment and management model for stream trout. River Res. Appl. 2021, 37, 1294–1302. [Google Scholar] [CrossRef]
- Piccolo, J.J.; Norrgård, J.R.; Greenberg, L.A.; Schmitz, M.; Bergman, E. Conservation of endemic landlocked salmonids in regulated rivers: A case-study from Lake Vänern, Sweden. Fish Fish. 2012, 13, 418–433. [Google Scholar] [CrossRef]
- Railsback, S.F.; Harvey, B.C.; Jackson, S.K.; Lamberson, R.H. InSTREAM: The Individual-Based Stream Trout Research and Environmental Assessment Model; (General Technical Report. PSW-GTR-218); USDA Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2009.
- Bjørnås, K.L.; Railsback, S.F.; Calles, O.; Piccolo, J.J. Modeling Atlantic salmon (Salmo salar) and brown trout (S. trutta) population responses and interactions under increased minimum flow in a regulated river. Ecol. Eng. 2021, 162, 106182. [Google Scholar] [CrossRef]
- Strömbäck, L.; Hjerdt, N.; Eriksson, L.B.; Lewau, P. Vattenwebb: A transparent service to support decision makers in achieving improved water status. In 10th IFIP WG 5.11 International Symposium, Proceedings of the ISESS 2013, Neusiedl Am See, Austria, 9–11 October 2013; Hřebíček, J., Schimak, G., Kubásek, M., Rizzoli, A.E., Eds.; IFIP International Federation for Information Processing: Laxenburg, Austria, 2013. [Google Scholar]
- Sundt-Hansen, L.E.; Hedger, R.D.; Ugedal, O.; Diserud, O.H.; Finstad, A.G.; Sauterleute, J.F.; Tøfte, L.; Alfredsen, K.; Forseth, T. Modelling climate change effects on Atlantic salmon: Implications for mitigation in regulated rivers. Sci. Total Environ. 2018, 631, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Crane, D.P.; Ogle, D.H.; Shoup, D.E. Use and misuse of a common growth metric: Guidance for appropriately calculating and reporting specific growth rate. Rev. Aquac. 2020, 12, 1542–1547. [Google Scholar] [CrossRef]
- Heard, S.B. Of Course You Can Do Significance Testing on Simulation Data! Scientist Sees Squirrel. 2019. Available online: https://scientistseessquirrel.wordpress.com/2019/05/07/of-course-you-can-do-significance-testing-on-simulation-data/ (accessed on 11 May 2023).
- Elliott, J.; Elliott, J.A. Temperature requirements of Atlantic salmon Salmo salar, brown trout Salmo trutta and Arctic charr Salvelinus alpinus: Predicting the effects of climate change. J. Fish Biol. 2010, 77, 1793–1817. [Google Scholar] [CrossRef]
- Watz, J.; Piccolo, J.; Bergman, E.; Greenberg, L. Day and night drift-feeding by juvenile salmonids at low water temperatures. Environ. Biol. Fishes 2014, 97, 505–513. [Google Scholar] [CrossRef]
- Hurst, T.P. Causes and consequences of winter mortality in fishes. J. Fish Biol. 2007, 71, 315–345. [Google Scholar] [CrossRef]
- Heggenes, J.; Gunnar Dokk, J. Contrasting temperatures, waterflows, and light: Seasonal habitat selection by young Atlantic salmon and brown trout in a boreonemoral river. Regul. Rivers Res. Manag. 2001, 17, 623–635. [Google Scholar] [CrossRef]
- Watz, J.; Otsuki, Y.; Nagatsuka, K.; Hasegawa, K.; Koizumi, I. Temperature-dependent competition between juvenile salmonids in small streams. Freshw. Biol. 2019, 64, 1534–1541. [Google Scholar] [CrossRef]
- Zillig, K.W.; Lusardi, R.A.; Cocherell, D.E.; Fangue, N.A. Interpopulation variation in thermal physiology among seasonal runs of Chinook salmon. Can. J. Fish. Aquat. Sci. 2023, 80, 1–13. [Google Scholar] [CrossRef]
- Hesthagen, T.; Larsen, B.M.; Bolstad, G.; Fiske, P.; Jonsson, B. Mitigation of acidified salmon rivers–effects of liming on young brown trout Salmo trutta. J. Fish Biol. 2017, 91, 1350–1364. [Google Scholar] [CrossRef]
- Piccolo, J.J.; Hughes, N.F.; Bryant, M.D. Water velocity influences prey detection and capture by drift-feeding juvenile coho salmon (Oncorhynchus kisutch) and steelhead (Oncorhynchus mykiss irideus). Can. J. Fish. Aquat. Sci. 2008, 65, 266–275. [Google Scholar] [CrossRef] [Green Version]
- Addo, L.; Hajiesmaeili, M.; Piccolo, J.J.; Watz, J. Growth and mortality of sympatric Atlantic salmon and brown trout fry in fluctuating and stable flows. Ecol. Freshw. Fish 2023, 32, 282–290. [Google Scholar] [CrossRef]



| Species | Starting Age | Number | Length (Min–Mode–Max; mm) |
|---|---|---|---|
| Atlantic salmon | one summer | 810 | 6.2–9.0–14.3 |
| two summers | 40 | 15.5–17.2–18.6 | |
| Brown trout | one summer | 360 | 4.6–10.5–19.0 |
| two summers | 20 | 19.7–22.5–23.5 |
| Variable | Population | Source of Variation | F | df | p | ηp2 |
|---|---|---|---|---|---|---|
| Specific | Atlantic salmon, one summer | Temperature | 112.65 | 1, 16 | <0.001 | 0.876 |
| growth | Flow | <0.01 | 1, 16 | 1.000 | <0.001 | |
| rate | Temperature × Flow | 0.67 | 1, 16 | 0.426 | 0.040 | |
| Brown trout, one summer | Temperature | 136.97 | 1, 16 | <0.001 | 0.895 | |
| Flow | 2.80 | 1, 16 | 0.114 | 0.149 | ||
| Temperature × Flow | 4.98 | 1, 16 | 0.040 | 0.237 | ||
| Atlantic salmon, two summers | Temperature | 13.13 | 1, 16 | 0.002 | 0.451 | |
| Flow | 0.04 | 1, 16 | 0.851 | 0.002 | ||
| Temperature × Flow | 1.78 | 1, 16 | 0.201 | 0.100 | ||
| Brown trout, two summers | Temperature | 8.08 | 1, 16 | 0.012 | 0.336 | |
| Flow | 1.29 | 1, 16 | 0.272 | 0.075 | ||
| Temperature × Flow | <0.01 | 1, 16 | 1.000 | <0.001 | ||
| Survival | Atlantic salmon, one summer | Temperature | 158.24 | 1, 16 | <0.001 | 0.908 |
| rate | Flow | 0.20 | 1, 16 | 0.661 | 0.012 | |
| Temperature × Flow | 11.84 | 1, 16 | 0.030 | 0.425 | ||
| Brown trout, one summer | Temperature | 7.26 | 1, 16 | 0.160 | 0.312 | |
| Flow | 0.10 | 1, 16 | 0.752 | 0.006 | ||
| Temperature × Flow | 5.29 | 1, 16 | 0.035 | 0.248 | ||
| Atlantic salmon, two summers | Temperature | 0.90 | 1, 16 | 0.358 | 0.053 | |
| Flow | 0.07 | 1, 16 | 0.800 | 0.004 | ||
| Temperature × Flow | 0.36 | 1, 16 | 0.555 | 0.022 | ||
| Brown trout, two summers | Temperature | 0.18 | 1, 16 | 0.679 | 0.011 | |
| Flow | 0.18 | 1, 16 | 0.679 | 0.011 | ||
| Temperature × Flow | 1.60 | 1, 16 | 0.224 | 0.091 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watz, J.; Schill, J.; Addo, L.; Piccolo, J.J.; Hajiesmaeili, M. Increased Temperature and Discharge Influence Overwinter Growth and Survival of Juvenile Salmonids in a Hydropeaking River: Simulating Effects of Climate Change Using Individual-Based Modelling. Fishes 2023, 8, 323. https://doi.org/10.3390/fishes8060323
Watz J, Schill J, Addo L, Piccolo JJ, Hajiesmaeili M. Increased Temperature and Discharge Influence Overwinter Growth and Survival of Juvenile Salmonids in a Hydropeaking River: Simulating Effects of Climate Change Using Individual-Based Modelling. Fishes. 2023; 8(6):323. https://doi.org/10.3390/fishes8060323
Chicago/Turabian StyleWatz, Johan, Joel Schill, Louis Addo, John J. Piccolo, and Mahboobeh Hajiesmaeili. 2023. "Increased Temperature and Discharge Influence Overwinter Growth and Survival of Juvenile Salmonids in a Hydropeaking River: Simulating Effects of Climate Change Using Individual-Based Modelling" Fishes 8, no. 6: 323. https://doi.org/10.3390/fishes8060323
APA StyleWatz, J., Schill, J., Addo, L., Piccolo, J. J., & Hajiesmaeili, M. (2023). Increased Temperature and Discharge Influence Overwinter Growth and Survival of Juvenile Salmonids in a Hydropeaking River: Simulating Effects of Climate Change Using Individual-Based Modelling. Fishes, 8(6), 323. https://doi.org/10.3390/fishes8060323