Conductive Nanofilms with Oppositely Charged Reduced Graphene Oxides as a Base for Electroactive Coatings and Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Graphene Oxide Characterization
2.2. Graphene Oxide Modification Procedure
2.3. Formation of Polyelectrolyte Films with Reduced Graphene Oxide Nanosheets
2.4. Characterization of Polyelectrolyte Films with Reduced Graphene Oxide
3. Results and Discussion
3.1. Characterization of Positively and Negatively Charged Graphene Oxide
3.2. Characterization of (GO+/GO−)n and (GO+/PSS)n Films
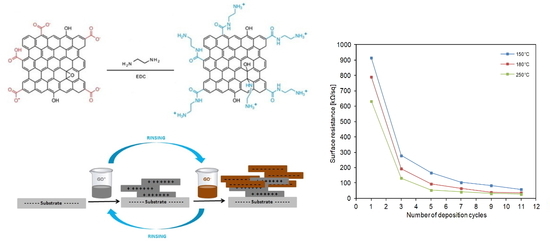
3.3. Conductivity of Multilayer Films (rGO+/rGO−, rGO+/PSS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Li, C.; Shi, G. Functional composite materials based on chemically converted graphene. Adv. Mater. 2011, 23, 1089–1115. [Google Scholar] [CrossRef]
- Peressi, M. Surface Functionalization of Graphene. In Graphene Chemistry: Theoretical Perspectives; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 233–253. ISBN 9781118691281. [Google Scholar]
- Vieira, N.C.S.; Borme, J.; MacHado, G.; Cerqueira, F.; Freitas, P.P.; Zucolotto, V.; Peres, N.M.R.; Alpuim, P. Graphene field-effect transistor array with integrated electrolytic gates scaled to 200 mm. J. Phys. Condens. Matter 2016, 28, 085302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef]
- Li, C.; Adamcik, J.; Mezzenga, R. Biodegradable nanocomposites of amyloid fibrils and graphene with shape-memory and enzyme-sensing properties. Nat. Nanotechnol. 2012, 7, 421–427. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.M. The reduction of graphene oxide. Carbon N. Y. 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Mao, S.; Pu, H.; Chen, J. Graphene oxide and its reduction: Modeling and experimental progress. RSC Adv. 2012, 2, 2643–2662. [Google Scholar] [CrossRef]
- Moon, I.K.; Lee, J.; Ruoff, R.S.; Lee, H. Reduced graphene oxide by chemical graphitization. Nat. Commun. 2010, 1, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Z.; Wang, K.; Wei, T.; Yan, J.; Song, L.; Shao, B. An environmentally friendly and efficient route for the reduction of graphene oxide by aluminum powder. Carbon N. Y. 2010, 48, 1686–1689. [Google Scholar] [CrossRef]
- Kuila, T.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Recent advances in the efficient reduction of graphene oxide and its application as energy storage electrode materials. Nanoscale 2013, 5, 52–71. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascón, J.M.D. Graphene oxide dispersions in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cote, L.J.; Kim, F.; Yuan, W.; Shull, K.R.; Huang, J. Graphene oxide sheets at interfaces. J. Am. Chem. Soc. 2010, 132, 8180–8186. [Google Scholar] [CrossRef] [PubMed]
- Kuziel, A.W.; Milowska, K.Z.; Chau, P.L.; Boncel, S.; Koziol, K.K.; Yahya, N.; Payne, M.C. The True Amphipathic Nature of Graphene Flakes: A Versatile 2D Stabilizer. Adv. Mater. 2020, 32, 2000608. [Google Scholar] [CrossRef]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- Faghani, A.; Donskyi, I.S.; Fardin Gholami, M.; Ziem, B.; Lippitz, A.; Unger, W.E.S.; Böttcher, C.; Rabe, J.P.; Haag, R.; Adeli, M. Controlled Covalent Functionalization of Thermally Reduced Graphene Oxide To Generate Defined Bifunctional 2D Nanomaterials. Angew. Chem. Int. Ed. 2017, 56, 2675–2679. [Google Scholar] [CrossRef] [PubMed]
- Güneş, F.; Shin, H.J.; Biswas, C.; Han, G.H.; Kim, E.S.; Chae, S.J.; Choi, J.Y.; Lee, Y.H. Layer-by-layer doping of few-layer graphene film. ACS Nano 2010, 4, 4595–4600. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, M.; Son, S.; Cho, S.Y.; Lee, S.; Won, D.K.; Ryu, J.; Bae, I.; Kim, H.M.; Kim, K.B. Sheet Resistance Analysis of Interface-Engineered Multilayer Graphene: Mobility Versus Sheet Carrier Concentration. ACS Appl. Mater. Interfaces 2020, 12, 30932–30940. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.S.; Geng, J.; Jung, H.T. Layer-by-layer assembly of graphene and gold nanoparticles by vacuum filtration and spontaneous reduction of gold ions. Chem. Commun. 2009, 2174–2176. [Google Scholar] [CrossRef]
- Hong, W.; Xu, Y.; Lu, G.; Li, C.; Shi, G. Transparent graphene/PEDOT-PSS composite films as counter electrodes of dye-sensitized solar cells. Electrochem. Commun. 2008, 10, 1555–1558. [Google Scholar] [CrossRef]
- Cote, L.J.; Kim, F.; Huang, J. Langmuir-blodgett assembly of graphite oxide single layers. J. Am. Chem. Soc. 2009, 131, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Milošević, I.R.; Vasić, B.; Matković, A.; Vujin, J.; Aškrabić, S.; Kratzer, M.; Griesser, T.; Teichert, C.; Gajić, R. Single-step fabrication and work function engineering of Langmuir-Blodgett assembled few-layer graphene films with Li and Au salts. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.-D. Buildup of ultrathin multilayer films by a self-assembly process, 1 consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surfaces. Makromol. Chem. Macromol. Symp. 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Cassagneau, T.; Guérin, F.; Fendler, J.H. Preparation and characterization of ultrathin films layer-by-layer self-assembled from graphite oxide nanoplatelets and polymers. Langmuir 2000, 16, 7318–7324. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Y.; Li, C.; Qin, C.; Shi, M.; Ye, M. Layer-by-layer self-assembly of graphene nanoplatelets. Langmuir 2009, 25, 6122–6128. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Hong, T.K.; Kang, D.; Lee, J.; Heo, M.; Kim, J.Y.; Kim, B.S.; Shin, H.S. Highly controllable transparent and conducting thin films using layer-by-layer assembly of oppositely charged reduced graphene oxides. J. Mater. Chem. 2011, 21, 3438–3442. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Hou, Y.; Kotov, N.A. Graphene-based multilayers: Critical evaluation of materials assembly techniques. Nano Today 2012, 7, 430–447. [Google Scholar] [CrossRef]
- Pajor-Świerzy, A.; Kruk, T.; Warszyński, P. Enhancement of the Electrocatalytic Properties of Prussian Blue Containing Multilayer Films Formed by Reduced Graphene Oxide. Colloids Interface Sci. Commun. 2014, 1, 6–9. [Google Scholar] [CrossRef] [Green Version]
- Kruk, T.; Socha, R.P.; Szyk-Warszyńska, L.; Warszyński, P. Flexible and ultrathin polyelectrolyte conductive coatings formed with reduced graphene oxide as a base for advanced new materials. Appl. Surf. Sci. 2019, 484, 501–510. [Google Scholar] [CrossRef]
- Kotov, N.A.; Dékány, I.; Fendler, J.H. Ultrathin graphite oxide-polyelectrolyte composites prepared by self-assembly: Transition between conductive and non-conductive states. Adv. Mater. 1996, 8, 637–641. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Bolling, L.; Priolo, M.A.; Grunlan, J.C. Graphene: Super Gas Barrier and Selectivity of Graphene Oxide-Polymer Multilayer Thin Films (Adv. Mater. 4/2013). Adv. Mater. 2013, 25, 493. [Google Scholar] [CrossRef]
- Chen, J.T.; Fu, Y.J.; An, Q.F.; Lo, S.C.; Huang, S.H.; Hung, W.S.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Tuning nanostructure of graphene oxide/polyelectrolyte LbL assemblies by controlling pH of GO suspension to fabricate transparent and super gas barrier films. Nanoscale 2013, 5, 9081–9088. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, H.; Kim, N.H.; Hui, D.; Lee, J.H.; Li, Q.; Sun, H.; Li, P. Preparation of graphene oxide/polyethyleneimine layer-by-layer assembled film for enhanced hydrogen barrier property. Compos. Part B Eng. 2016, 92, 252–258. [Google Scholar] [CrossRef]
- Gross, M.A.; Sales, M.J.A.; Soler, M.A.G.; Pereira-Da-Silva, M.A.; Da Silva, M.F.P.; Paterno, L.G. Reduced graphene oxide multilayers for gas and liquid phases chemical sensing. RSC Adv. 2014, 4, 17917–17924. [Google Scholar] [CrossRef]
- do Santos, F.A.; Vieira, N.C.S.; Zambianco, N.A.; Janegitz, B.C.; Zucolotto, V. The layer-by-layer assembly of reduced graphene oxide films and their application as solution-gated field-effect transistors. Appl. Surf. Sci. 2021, 543, 148698. [Google Scholar] [CrossRef]
- Lee, T.; Min, S.H.; Gu, M.; Jung, Y.K.; Lee, W.; Lee, J.U.; Seong, D.G.; Kim, B.S. Layer-by-Layer Assembly for Graphene-Based Multilayer Nanocomposites: Synthesis and Applications. Chem. Mater. 2015, 27, 3785–3796. [Google Scholar] [CrossRef]
- Kim, S.M.; Joo, P.; Ahn, G.; Cho, I.H.; Kim, D.H.; Song, W.K.; Kim, B.S.; Yoon, M.H. Transparent conducting films based on reduced graphene oxide multilayers for biocompatible neuronal interfaces. J. Biomed. Nanotechnol. 2013, 9, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Jijie, R.; Kahlouche, K.; Barras, A.; Yamakawa, N.; Bouckaert, J.; Gharbi, T.; Szunerits, S.; Boukherroub, R. Reduced graphene oxide/polyethylenimine based immunosensor for the selective and sensitive electrochemical detection of uropathogenic Escherichia coli. Sens. Actuators B Chem. 2018, 260, 255–263. [Google Scholar] [CrossRef]
- Zhu, J.; He, J. Assembly and benign step-by-step post-treatment of oppositely charged reduced graphene oxides for transparent conductive thin films with multiple applications. Nanoscale 2012, 4, 3558–3566. [Google Scholar] [CrossRef]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhi, L.; Tsao, N.; Tomović, Ž.; Li, J.; Müllen, K. Transparent carbon films as electrodes in organic solar cells. Angew. Chem. Int. Ed. 2008, 47, 2990–2992. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Eda, G.; Mattevi, C.; Kim, H.K.; Chhowalla, M. Highly uniform 300 mm wafer-scale deposition of single and multilayered chemically derived graphene thin films. ACS Nano 2010, 4, 524–528. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Cho, S.M.; Kim, W.J.; Park, J.; Yoo, P.J. Fabrication of graphene thin films based on layer-by-layer self-assembly of functionalized graphene nanosheets. ACS Appl. Mater. Interfaces 2011, 3, 360–368. [Google Scholar] [CrossRef]
- Hwang, H.; Joo, P.; Kang, M.S.; Ahn, G.; Han, J.T.; Kim, B.S.; Cho, J.H. Highly tunable charge transport in layer-by-layer assembled graphene transistors. ACS Nano 2012, 6, 2432–2440. [Google Scholar] [CrossRef]
- Helmenstine, A.M.P. Resistivity Table/Chart for Common Materials|Electronics Notes. Available online: https://www.electronics-notes.com/articles/basic_concepts/resistance/electrical-resistivity-table-materials.php (accessed on 9 March 2021).
- Overview of Materials for Polyimide. Available online: http://www.matweb.com/search/DataSheet.aspx?MatGUID=ab35b368ab9c40848f545c35bdf1a672&ckck=1 (accessed on 9 March 2021).
- Cha, M.J.; Song, W.; Kim, Y.; Jung, D.S.; Jung, M.W.; Lee, S.I.; Adhikari, P.D.; An, K.S.; Park, C.Y. Long-term air-stable n-type doped graphene by multiple lamination with polyethyleneimine. RSC Adv. 2014, 4, 37849–37853. [Google Scholar] [CrossRef]








| Film | Surface Resistance [kΩ/sq] Reduction Temp. 150 °C | Surface Resistance [kΩ/sq] Reduction Temp. 180 °C | Surface Resistance [kΩ/sq] Reduction Temp. 250 °C |
|---|---|---|---|
| (rGO+/rGO−)7 | 104 | 65 | 42 |
| (rGO+/rGO−)9 | 84 | 40 | 31 |
| (rGO+/rGO−)11 | 59 | 37 | 27 |
| Film | Surface Resistance [kΩ/sq] Reduction Temp. 150 °C | Surface Resistance [kΩ/sq] Reduction Temp. 180 °C | Surface Resistance [kΩ/sq] Reduction Temp. 250 °C |
|---|---|---|---|
| (rGO+/rGO−)7 | 190 | 49 | 37 |
| (rGO+/rGO−)9 | 123 | 34 | 27 |
| (rGO+/rGO−)11 | 74 | 22 | 15 |
| Film | Surface Resistance [kΩ/sq] Reduction Temp. 150 °C | Surface Resistance [kΩ/sq] Reduction Temp. 180 °C | Surface Resistance [kΩ/sq] Reduction Temp. 250 °C |
|---|---|---|---|
| (rGO+/rGO−)5 | 166 | 94 | 54 |
| (PEI/rGO−)11 | 79 | 49 | 37 |
| (rGO+/PSS)11 | 190 | 123 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruk, T.; Warszyński, P. Conductive Nanofilms with Oppositely Charged Reduced Graphene Oxides as a Base for Electroactive Coatings and Sensors. Colloids Interfaces 2021, 5, 20. https://doi.org/10.3390/colloids5020020
Kruk T, Warszyński P. Conductive Nanofilms with Oppositely Charged Reduced Graphene Oxides as a Base for Electroactive Coatings and Sensors. Colloids and Interfaces. 2021; 5(2):20. https://doi.org/10.3390/colloids5020020
Chicago/Turabian StyleKruk, Tomasz, and Piotr Warszyński. 2021. "Conductive Nanofilms with Oppositely Charged Reduced Graphene Oxides as a Base for Electroactive Coatings and Sensors" Colloids and Interfaces 5, no. 2: 20. https://doi.org/10.3390/colloids5020020
APA StyleKruk, T., & Warszyński, P. (2021). Conductive Nanofilms with Oppositely Charged Reduced Graphene Oxides as a Base for Electroactive Coatings and Sensors. Colloids and Interfaces, 5(2), 20. https://doi.org/10.3390/colloids5020020