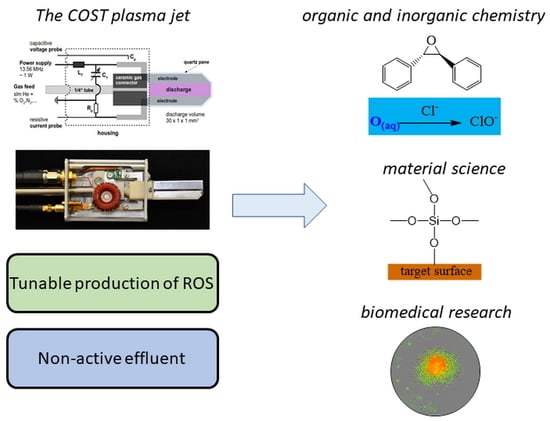
Applications of the COST Plasma Jet: More than a Reference Standard
Abstract
:1. Introduction
2. Applications of the COST Plasma Jet
2.1. Interaction with Organic Polymers
2.1.1. Photoresist Removal
2.1.2. Studying the Main Agents Used in Polymer Surface Modification
2.2. Preparation of Silicon-Based Films
2.3. COST Jet for Inorganic and Organic Chemistry
2.3.1. Studying the Reaction between Atomic O and Cl− in the Liquid Phase
2.3.2. Epoxidation of Trans-Stilbene with Atomic O
2.4. Biomedical Research
2.4.1. Studying Bactericidal Effects of CAPs
2.4.2. Identifying Optimal Parameters for Anti-CANCER treatments
3. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adamovich, I.; Baalrud, S.D.; Bogaerts, A.; Bruggeman, P.J.; Cappelli, M.; Colombo, V.; Czarnetzki, U.; Ebert, U.; Eden, J.G.; Favia, P.; et al. The 2017 Plasma Roadmap: Low temperature plasma science and technology. J. Phys. D Appl. Phys. 2017, 50, 323001. [Google Scholar] [CrossRef]
- Nikiforov, A.; Chen, Z. (Eds.) Atmospheric Pressure Plasma—From Diagnostics to Applications; InTechOpen: London, UK, 2019. [Google Scholar]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma-liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Wardenier, N.; Gorbanev, Y.; Van Moer, I.; Nikiforov, A.; Van Hulle, S.; Surmont, P.; Lynen, F.; Leys, C.; Bogaerts, A.; Vanraes, P. Removal of alachlor in water by non-thermal plasma: Reactive species and pathways in batch and continuous process. Water Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Bogaerts, A.; Neyts, E.C. Plasma Technology: An Emerging Technology for Energy Storage. ACS Energy Lett. 2018, 3, 1013–1027. [Google Scholar] [CrossRef] [Green Version]
- Laroussi, M.; Akan, T. Arc-Free Atmospheric Pressure Cold Plasma Jets: A Review. Plasma Process. Polym. 2007, 4, 777–788. [Google Scholar] [CrossRef]
- Winter, J.; Brandenburg, R.; Weltmann, K.-D. Atmospheric pressure plasma jets: An overview of devices and new directions. Plasma Sources Sci. Technol. 2015, 24, 064001. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef] [Green Version]
- Laroussi, M. Plasma Medicine: A Brief Introduction. Plasma 2018, 1, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Gorbanev, Y.; O’Connell, D.; Chechik, V. Non-Thermal Plasma in Contact with Water: The Origin of Species. Chem. Eur. J. 2016, 22, 3496–3505. [Google Scholar] [CrossRef] [Green Version]
- Reuter, S.; von Woedtke, T.; Weltmann, K.-D. The kINPen—A review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef]
- Attri, P.; Kumar, N.; Park, J.H.; Yadav, D.K.; Choi, S.; Uhm, H.S.; Kim, I.T.; Choi, F.H.; Lee, W. Influence of reactive species on the modification of biomolecules generated from the soft plasma. Sci. Rep. 2015, 5, 8221. [Google Scholar] [CrossRef] [PubMed]
- Gorbanev, Y.; Privat-Maldonado, A.; Bogaerts, A. Analysis of short-lived reactive species in plasma-air-water systems: The dos and the do nots. Anal. Chem. 2018, 90, 13151–13158. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.L.; Kong, M.G. Contrasting characteristics of linear-field and cross-field atmospheric plasma jets. Appl. Phys. Lett. 2008, 93, 111501. [Google Scholar] [CrossRef] [Green Version]
- MP1101—Biomedical Applications of Atmospheric Pressure Plasma Technology, European Cooperation in Science and Technology (COST). Available online: https://www.cost.eu/actions/MP1101 (accessed on 30 June 2019).
- Knake, N.; Schulz-von der Gathen, V. Investigations of the spatio-temporal build-up of atomic oxygen inside the micro-scaled atmospheric pressure plasma jet. Eur. Phys. J. D 2010, 60, 645–652. [Google Scholar] [CrossRef]
- Hemke, T.; Wollny, A.; Gebhardt, M.; Brinkmann, R.P.; Mussenbrock, T. Spatially resolved simulation of a radio-frequency driven micro-atmospheric pressure plasma jet and its effluent. J. Phys. D Appl. Phys. 2010, 44, 285206. [Google Scholar] [CrossRef]
- Golda, J.; Held, J.; Redeker, B.; Konkowski, M.; Beijer, P.; Sobota, A.; Kroesen, G.; Braithwaite, N.S.J.; Reuter, S.; Turner, M.M.; et al. Concepts and characteristics of the ‘COST Reference Microplasma Jet’. J. Phys. D Appl. Phys. 2016, 49, 08400. [Google Scholar] [CrossRef]
- Schneider, S.; Jarzina, F.; Lackmann, J.-W.; Golda, J.; Layes, V.; Schulz-von der Gathen, V.; Bandow, J.E.; Benedikt, J. Summarizing results on the performance of a selective set of atmospheric plasma jets for separation of photons and reactive particles. J. Phys. D Appl. Phys. 2015, 48, 444001. [Google Scholar] [CrossRef]
- Murakami, T.; Niemi, K.; Gans, T.; O’Connell, D.; Graham, W.G. Interacting kinetics of neutral and ionic species in an atmospheric-pressure helium–oxygen plasma with humid air impurities. Plasma Sources Sci. Technol. 2013, 22, 045010. [Google Scholar] [CrossRef]
- Murakami, T.; Niemi, K.; Gans, T.; O’Connell, D.; Graham, W.G. Afterglow chemistry of atmospheric-pressure helium–oxygen plasmas with humid air impurity. Plasma Sources Sci. Technol. 2014, 23, 025005. [Google Scholar] [CrossRef]
- Schröter, S.; Wijaikhum, A.; Gibson, A.R.; West, A.; Davies, H.L.; Minesi, N.; Dedrick, J.; Wagenaars, E.; de Oliveira, N.; Nahon, L.; et al. Chemical kinetics in an atmospheric pressure helium plasma containing humidity. Phys. Chem. Chem. Phys. 2018, 20, 24263–24286. [Google Scholar] [CrossRef] [Green Version]
- Dedrick, J.; Schröter, S.; Niemi, K.; Wijaikhum, A.; Wagenaars, E.; de Oliveira, N.; Nahon, L.; Booth, J.-P.; O’Connell, D.; Gans, T. Controlled production of atomic oxygen and nitrogen in a pulsed radio-frequency atmospheric-pressure plasma. J. Phys. D Appl. Phys. 2017, 50, 455204. [Google Scholar] [CrossRef]
- Ellerweg, D.; von Keudell, A.; Benedikt, J. Unexpected O and O3 production in the effluent of He/O2 microplasma jets emanating into ambient air. Plasma Sources Sci. Technol. 2012, 21, 034019. [Google Scholar] [CrossRef]
- Gorbanev, Y.; Van der Paal, J.; Van Boxem, W.; Dewilde, S.; Bogaerts, A. Reaction of chloride anion with atomic oxygen in aqueous solutions: Can cold plasma help in chemistry research? Phys. Chem. Chem. Phys. 2019, 21, 4117–4121. [Google Scholar] [CrossRef] [PubMed]
- Oehrlein, G.S.; Phaneuf, R.J.; Graves, D.B. Plasma-polymer interactions: A review of progress in understanding polymer resist mask durability during plasma etching for nanoscale fabrication. J. Vac. Sci. Technol. B 2011, 29, 010801. [Google Scholar] [CrossRef]
- West, A.; van der Schans, M.; Xu, C.; Cooke, M.; Wagenaars, E. Fast, downstream removal of photoresist using reactive oxygen species from the effluent of an atmospheric pressure plasma jet. Plasma Sources Sci. Technol. 2016, 25, 02LT01. [Google Scholar] [CrossRef]
- Hefny, M.M.; Nečas, D.; Zajíčková, L.; Benedikt, J. The transport and surface reactivity of O atoms during the atmospheric plasma etching of hydrogenated amorphous carbon films. Plasma Sources Sci. Technol. 2019, 28, 035010. [Google Scholar] [CrossRef]
- Rezaei, F.; Gorbanev, Y.; Chys, M.; Nikiforov, A.; Van Hulle, S.W.H.; Cos, P.; Bogaerts, A.; De Geyter, N. Investigation of plasma-induced chemistry in organic solutions for enhanced electrospun PLA nanofibers. Plasma Process. Polym. 2018, 15, 1700226. [Google Scholar] [CrossRef]
- Iqbal, M.; Dinh, D.K.; Abbas, Q.; Imran, M.; Sattar, H.; Ahmad, A.U. Controlled Surface Wettability by Plasma Polymer Surface Modification. Surfaces 2019, 2, 349–371. [Google Scholar] [CrossRef] [Green Version]
- Shaw, D.; West, A.; Bredin, J.; Wagenaars, E. Mechanisms behind surface modification of polypropylene film using an atmospheric-pressure plasma jet. Plasma Sources Sci. Technol. 2016, 25, 065018. [Google Scholar] [CrossRef]
- Reuter, R.; Ellerweg, D.; von Keudell, A.; Benedikt, J. Surface reactions as carbon removal mechanism in deposition of silicon dioxide films at atmospheric pressure. Appl. Phys. Lett. 2011, 98, 111502. [Google Scholar] [CrossRef]
- Kasuya, M.; Yasui, S.; Noda, M. Deposition of SiO2 Thin Films on Polycarbonate by Atmospheric-Pressure Plasma. Jpn. J. Appl. Phys. 2012, 51, 01AC01. [Google Scholar] [CrossRef]
- Reuter, R.; Rügner, K.; Ellerweg, D.; de los Arcos, T.; von Keudell, A.; Benedikt, J. The Role of Oxygen and Surface Reactions in the Deposition of Silicon Oxide Like Films from HMDSO at Atmospheric Pressure. Plasma Process. Polym. 2012, 9, 1116–1124. [Google Scholar] [CrossRef]
- Rügner, K.; Reuter, R.; Ellerweg, D.; de los Arcos, T.; von Keudell, A.; Benedikt, J. Insight into the Reaction Scheme of SiO2 Film Deposition at Atmospheric Pressure. Plasma Process. Polym. 2013, 10, 1061–1073. [Google Scholar] [CrossRef]
- Hefny, M.M.; Pattyn, C.; Lukes, P.; Benedikt, J. Atmospheric plasma generates oxygen atoms as oxidizing species in aqueous solutions. J. Phys. D Appl. Phys. 2016, 49, 404002. [Google Scholar] [CrossRef]
- Benedikt, J.; Hefny, M.M.; Shaw, A.; Buckley, B.R.; Iza, F.; Schäkermann, S.; Bandow, J.E. The fate of plasma-generated oxygen atoms in aqueous solutions: Non-equilibrium atmospheric pressure plasmas as an efficient source of atomic O(aq). Phys. Chem. Chem. Phys. 2018, 20, 12037–12042. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Gorbanev, Y.; De Backer, J.; Van Loenhout, J.; Van Boxem, W.; Lemière, F.; Cos, P.; Dewilde, S.; Smits, E.; Bogaerts, A. Non-Thermal Plasma as a Unique Delivery System of Short-Lived Reactive Oxygen and Nitrogen Species for Immunogenic Cell Death in Melanoma Cells. Adv. Sci. 2019, 6, 1802062. [Google Scholar] [CrossRef] [PubMed]
- Kondeti, V.S.S.K.; Phan, C.Q.; Wende, K.; Jablonowski, H.; Gangal, U.; Granick, J.L.; Hunter, R.C.; Bruggeman, P.J. Long-lived and short-lived reactive species produced by a cold atmospheric pressure plasma jet for the inactivation of Pseudomonas aeruginosa and Staphylococcus aureus. Free Radic. Biol. Med. 2018, 124, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Jirásek, V.; Lukeš, P. Formation of reactive chlorine species in saline solution treated by non-equilibrium atmospheric pressure He/O2 plasma jet. Plasma Sources Sci. Technol. 2019, 28, 035015. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, C.; Hu, S.; Chen, Y.; Jia, X.; Lau, R.; Yang, Y. Epoxidation of trans-stilbene and cis-cyclooctene over mesoporous vanadium catalysts: Support composition and pore structure effect. Appl. Catal. A Gen. 2012, 419, 194–202. [Google Scholar] [CrossRef]
- Gorbanev, Y.; Leifert, D.; Studer, A.; O’Connell, D.; Chechik, V. Initiating radical reactions with non-thermal plasmas. Chem. Commun. 2017, 53, 3685–3688. [Google Scholar] [CrossRef] [Green Version]
- Iza, F. Plasma-Driven Organic Synthesis: Waste-Free Epoxidation. In Proceedings of the 24th International Symposium on Plasma Chemistry, Naples, Italy, 9–14 July 2019. [Google Scholar]
- Privat-Maldonado, A.; Gorbanev, Y.; O’Connell, D.; Vann, R.; Chechik, V.; van der Woude, M.W. Non-target biomolecules alter macromolecular changes induced by bactericidal low-temperature plasma. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 2, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977–15995. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.B. Mechanisms of Plasma Medicine: Coupling Plasma Physics, Biochemistry, and Biology. IEEE Trans. Radiat. Plasma Med. Sci. 2017, 1, 281–292. [Google Scholar] [CrossRef]
- Gorbanev, Y.; Soriano, R.; O’Connell, D.; Chechik, V. An atmospheric pressure plasma setup to investigate the reactive species formation. J. Vis. Exp. 2016, 117, e54765. [Google Scholar] [CrossRef] [PubMed]
- Gorbanev, Y.; Verlackt, C.C.W.; Tinck, S.; Tuenter, E.; Foubert, K.; Cos, P.; Bogaerts, A. Combining experimental and modelling approaches to study the sources of reactive species induced in water by the COST RF plasma jet. Phys. Chem. Chem. Phys. 2018, 20, 2797–2808. [Google Scholar] [CrossRef]
- Lackmann, J.-W.; Schneider, S.; Edengeiser, E.; Jarzina, F.; Brinckmann, S.; Steinborn, E.; Havenith, M.; Benedikt, J.; Bandow, J.E. Photons and particles emitted from cold atmospheric-pressure plasma inactivate bacteria and biomolecules independently and synergistically. J. R. Soc. Interface 2013, 10, 20130591. [Google Scholar] [CrossRef] [Green Version]
- Lackmann, J.-W.; Wende, K.; Verlackt, C.; Golda, J.; Volzke, J.; Kogelheide, F.; Held, J.; Bekeschus, S.; Bogaerts, A.; Schulz-von der Gathen, V.; et al. Chemical fingerprints of cold physical plasmas—An experimental and computational study using cysteine as tracer compound. Sci. Rep. 2018, 8, 7736. [Google Scholar] [CrossRef]
- Vermeylen, S.; De Waele, J.; Vanuytsel, S.; De Backer, J.; Van der Paal, J.; Ramakers, M.; Leyssens, K.; Marcq, E.; Van Audenaerde, J.; Smits, E.L.J.; et al. Cold atmospheric plasma treatment of melanoma and glioblastoma cancer cells. Plasma Process. Polym. 2016, 13, 1195–1205. [Google Scholar] [CrossRef]
- Van der Paal, J.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem. Sci. 2016, 7, 489–498. [Google Scholar] [CrossRef]
- Bekeschus, S.; Wende, K.; Hefny, M.M.; Rödder, K.; Jablonowski, H.; Schmidt, A.; von Woedtke, T.; Weltmann, K.-D.; Benedikt, J. Oxygen atoms are critical in rendering THP-1 leukaemia cells susceptible to cold physical plasma-induced apoptosis. Sci. Rep. 2016, 7, 2791. [Google Scholar] [CrossRef]
- Keidar, M. A prospectus on innovations in the plasma treatment of cancer. Phys. Plasmas 2018, 25, 083504. [Google Scholar] [CrossRef]
- Heirman, P.; Van Boxem, W.; Bogaerts, A. Reactivity and stability of plasma-generated oxygen and nitrogen species in buffered water solution: A computational study. Phys. Chem. Chem. Phys. 2019, 21, 12881–12894. [Google Scholar] [CrossRef] [PubMed]
- Privat-Maldonado, A.; Gorbanev, Y.; Dewilde, S.; Smits, E.; Bogaerts, A. Reduction of human glioblastoma spheroids using cold atmospheric plasma: The combined effect of short- and long-lived reactive species. Cancers 2018, 10, 394. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorbanev, Y.; Golda, J.; Gathen, V.S.-v.d.; Bogaerts, A. Applications of the COST Plasma Jet: More than a Reference Standard. Plasma 2019, 2, 316-327. https://doi.org/10.3390/plasma2030023
Gorbanev Y, Golda J, Gathen VS-vd, Bogaerts A. Applications of the COST Plasma Jet: More than a Reference Standard. Plasma. 2019; 2(3):316-327. https://doi.org/10.3390/plasma2030023
Chicago/Turabian StyleGorbanev, Yury, Judith Golda, Volker Schulz-von der Gathen, and Annemie Bogaerts. 2019. "Applications of the COST Plasma Jet: More than a Reference Standard" Plasma 2, no. 3: 316-327. https://doi.org/10.3390/plasma2030023
APA StyleGorbanev, Y., Golda, J., Gathen, V. S. -v. d., & Bogaerts, A. (2019). Applications of the COST Plasma Jet: More than a Reference Standard. Plasma, 2(3), 316-327. https://doi.org/10.3390/plasma2030023