Nutrient Storage and Stoichiometry of the Forest Floor Organic Matter in Japanese Forests
Abstract
:1. Introduction
2. Materials and Methods
3. Results
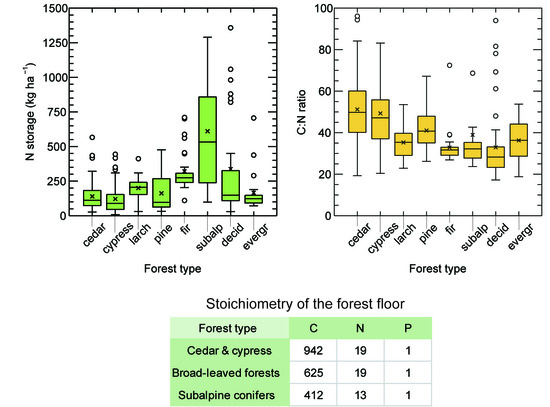
3.1. Dry Mass and Nutrient Storage in Predominant Forest Type
3.2. Total Nutrient Concentrations in the Forest Floor
3.3. Stoichiometry of Carbon and Nutrients in the Forest Floor
3.4. Relationship between the Dry Weight of the Forest Floor and Nutrient Storage
4. Discussion
4.1. Forest Floor Mass
4.2. Nutrient Storage of Nitrogen and Phosphorus
4.3. Mineral Storage in the Forest Floor
4.4. Stoichiometry
4.5. Correlation between Dry Weight of the Forest Floor and Nutrient Storage
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Element | Forest Type | a | b | R2 | n |
|---|---|---|---|---|---|
| Nitrogen | Cedar | 0.916 | 1.063 | 0.803 | 98 |
| Cypress | 0.899 | 1.083 | 0.871 | 52 | |
| Larch | 1.053 | 1.031 | 0.893 | 16 | |
| Pine | 0.941 | 1.089 | 0.924 | 21 | |
| Todo fir | 1.038 | 1.060 | 0.803 | 31 | |
| Subalpine coniferous | 1.050 | 1.045 | 0.755 | 25 | |
| Deciduous | 1.110 | 1.048 | 0.864 | 49 | |
| Evergreen | 1.275 | 0.839 | 0.697 | 22 | |
| Phosphorus | Cedar | −0.371 | 1.054 | 0.443 | 43 |
| Cypress | −0.359 | 1.003 | 0.598 | 26 | |
| Pine | −0.047 | 0.920 | 0.903 | 10 | |
| Todo fir | −0.397 | 1.337 | 0.709 | 30 | |
| Subalpine coniferous | 0.089 | 0.923 | 0.535 | 18 | |
| Deciduous | 0.233 | 0.678 | 0.547 | 30 | |
| Evergreen | −0.454 | 1.202 | 0.725 | 14 | |
| Potassium | Cedar | −0.093 | 1.107 | 0.547 | 96 |
| Cypress | 0.124 | 1.004 | 0.609 | 46 | |
| Larch | −0.087 | 1.198 | 0.682 | 16 | |
| Pine | 0.343 | 0.810 | 0.753 | 15 | |
| Todo fir | 0.277 | 0.965 | 0.700 | 29 | |
| Subalpine coniferous | 0.633 | 0.680 | 0.672 | 13 | |
| Deciduous | −0.372 | 1.200 | 0.348 | 33 | |
| Evergreen | 0.223 | 0.890 | 0.581 | 16 | |
| Calcium | Cedar | 1.292 | 0.840 | 0.646 | 96 |
| Cypress | 0.949 | 0.836 | 0.676 | 44 | |
| Larch | 0.878 | 0.958 | 0.613 | 16 | |
| Pine | 1.134 | 0.645 | 0.631 | 15 | |
| Todo fir | 0.063 | 1.603 | 0.574 | 27 | |
| Subalpine coniferous | 1.328 | 0.668 | 0.393 | 12 | |
| Deciduous | 1.248 | 0.522 | 0.128 | 33 | |
| Evergreen | 1.705 | 0.397 | 0.102 | 16 | |
| Magnesium | Cedar | −0.003 | 1.148 | 0.612 | 92 |
| Cypress | 0.166 | 0.382 | 0.567 | 41 | |
| Larch | 0.353 | 0.853 | 0.576 | 15 | |
| Pine | 0.634 | 0.567 | 0.538 | 15 | |
| Deciduous | −0.117 | 1.083 | 0.381 | 32 | |
| Evergreen | 0.694 | 0.595 | 0.209 | 16 |
References
- IPCC. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Prepared by National Greenhouse Gas Inventories Programme; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; IGES: Kanagawa, Japan, 2006; ISBN 978-4-88788-032-0. [Google Scholar]
- Takahashi, M. Comparison of nutrient concentrations in organic layers between broad-leaved and coniferous forests. Soil Sci. Plant Nutr. 1997, 43, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Yim, J.S.; Son, Y.M.; Son, Y.; Kim, R. Estimation of Forest Carbon Stocks for National Greenhouse Gas Inventory Reporting in South Korea. Forests 2018, 9, 625. [Google Scholar] [CrossRef] [Green Version]
- Domke, G.M.; Perry, C.; Walters, B.; Woodall, C.W.; Russell, M.B.; Smith, J.E. Estimating litter carbon stocks on forest land in the United States. Sci. Total Environ. 2016, 557–558, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.J.; Yim, M.H.; Nakane, K. Contribution of microarthropods to the decomposition of needle litter in a Japanese cedar (Cryptomeria japonica D. Don) plantation. For. Ecol. Manag. 2006, 234, 192–198. [Google Scholar] [CrossRef]
- Petersen, H.; Luxton, M. A Comparative Analysis of Soil Fauna Populations and Their Role in Decomposition Processes. Oikos 1982, 39, 288. [Google Scholar] [CrossRef]
- Saitoh, S.; Fujii, S.; Takeda, H. Evaluation of the bottom-up force of accumulated organic matter on microarthropods in a temperate forest floor. Eur. J. Soil Biol. 2011, 47, 409–413. [Google Scholar] [CrossRef]
- Li, X.; Yin, X.; Wang, Z.; Fan, W. Interaction between decomposing litter and soil fauna of the Betula ermanii forest floor of the Changbai Mountains, China. Can. J. For. Res. 2014, 44, 1507–1514. [Google Scholar] [CrossRef]
- Gosz, J.R.; Likens, G.E.; Bormann, F.H. Organic matter and nutrient dynamics of the forest and forest floor in the Hubbard Brook Forest. Oecologia 1976, 22, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Sudo, R. Role of litter in acid buffer capacity in forest area. Environ. Sci. 1997, 10, 11–19. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.; Yu, M.; Cao, N.; Yan, J. Soil organic matter is important for acid buffering and reducing aluminum leaching from acidic forest soils. Chem. Geol. 2018, 501, 86–94. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 978-3-030-59630-9. [Google Scholar]
- Osono, T.; Takeda, H. Decomposition of organic chemical components in relation to nitrogen dynamics in leaf litter of 14 tree species in a cool temperate forest. Ecol. Res. 2004, 20, 41–49. [Google Scholar] [CrossRef]
- Aber, J.D.; Melillo, J.M.; McClaugherty, C.A. Predicting long-term patterns of mass loss, nitrogen dynamics, and soil organic matter formation from initial fine litter chemistry in temperate forest ecosystems. Can. J. Bot. 1990, 68, 2201–2208. [Google Scholar] [CrossRef] [Green Version]
- Yue, K.; Ni, X.; Fornara, D.A.; Peng, Y.; Liao, S.; Tan, S.; Wang, D.; Wu, F.; Yang, Y. Dynamics of Calcium, Magnesium, and Manganese During Litter Decomposition in Alpine Forest Aquatic and Terrestrial Ecosystems. Ecosystems 2020, 24, 516–529. [Google Scholar] [CrossRef]
- Kaneko, N.; Katagiri, N.; Miyake, N. Decomposition process of needle litter of Japanese Red Cedar (Cryptomeria japonica) by oribatid mites. J. Jpn. For. Soc. 1990, 72, 158–162. [Google Scholar] [CrossRef]
- Ge, X.; Zeng, L.; Xiao, W.; Huang, Z.; Geng, X.; Tan, B. Effect of litter substrate quality and soil nutrients on forest litter decomposition: A review. Acta Ecol. Sin. 2013, 33, 102–108. [Google Scholar] [CrossRef]
- Takahashi, M.; Matoba, S.; Sato, T. Size distribution and concentration of carbon and nitrogen in each size class of organic matter in the organic layers under a Pinus pumila stand. Jpn. J. For. Environ. 1996, 38, 109–114. [Google Scholar] [CrossRef]
- Kanazawa, S.; Wada, H.; Takesima, S.; Takai, Y. The decomposition processes and existence forms of organic matter in subalpine forest soil (Part 1): Microscopic observation and carbon-nitrogen content of fractionated organic layer of Pwh soil type of Mt. Shigayama. Jpn. J. Soil Sci. Plant Nutr. 1977, 48, 181–186. [Google Scholar] [CrossRef]
- Vogt, K.A.; Grier, C.C.; Meier, C.E.; Keyes, M.R. Organic Matter and Nutrient Dynamics in Forest Floors of Young and Mature Abies amabilis Stands in Western Washington, as Affected by Fine-Root Input. Ecol. Monogr. 1983, 53, 139–157. [Google Scholar] [CrossRef]
- Carnol, M.; Bazgir, M. Nutrient return to the forest floor through litter and throughfall under 7 forest species after conversion from Norway spruce. For. Ecol. Manag. 2013, 309, 66–75. [Google Scholar] [CrossRef]
- Tobón, C.; Sevink, J.; Verstraten, J.M. Litterflow chemistry and nutrient uptake from the forest floor in northwest Amazonian Forest ecosystems. Biogeochemistry 2004, 69, 315–339. [Google Scholar] [CrossRef]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Penuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef] [Green Version]
- Moore, T.R.; Trofymow, J.A.; Prescott, C.E.; Fyles, J.; Titus, B.D. Patterns of Carbon, Nitrogen and Phosphorus Dynamics in Decomposing Foliar Litter in Canadian Forests. Ecosystems 2006, 9, 46–62. [Google Scholar] [CrossRef]
- Wen-Ji, M.; Yan-Tao, Z.; Qing-Qing, Z.; Arshad, A.; Qing-Ru, S.; En-Rong, A.Y. C:N:P stoichiometry in forest floor litter of evergreen broad-leaved forests at different successional stages in Tiantong, Zhejiang, eastern China. Chin. J. Plant Ecol. 2014, 38, 833–842. [Google Scholar] [CrossRef]
- Tsukada, M. Vegetation and Climate during the Last Glacial Maximum in Japan. Quat. Res. 1983, 19, 212–235. [Google Scholar] [CrossRef]
- Nakashizuka, T.; Iida, S. Composition, dynamics and disturbance regime of temperate deciduous forests in Monsoon Asia. Vegetatio 1995, 121, 23–30. [Google Scholar] [CrossRef]
- Covington, W.W. Changes in Forest Floor Organic Matter and Nutrient Content Following Clear Cutting in Northern Hardwoods. Ecology 1981, 62, 41–48. [Google Scholar] [CrossRef]
- Zajícová, K.; Chuman, T. Spatial variability of forest floor and topsoil thicknesses and their relation to topography and forest stand characteristics in managed forests of Norway spruce and European beech. Eur. J. For. Res. 2020, 140, 77–90. [Google Scholar] [CrossRef]
- Tokuchi, N.; Takeda, H.; Yoshida, K.; Iwatsubo, G. Topographical variations in a plant-soil system along a slope on Mt Ryuoh, Japan. Ecol. Res. 1999, 14, 361–369. [Google Scholar] [CrossRef]
- Takahashi, M. Direct estimation of carbon mass of organic layer from dry weight. J. For. Res. 2005, 10, 239–241. [Google Scholar] [CrossRef]
- Morita, K. Mineral composition of the fresh litter of major tree species in Japan. Bull. Gov. For. Exp. Sta. 1972, 243, 33–50. [Google Scholar]
- Takahashi, M.; Ishizuka, S.; Ugawa, S.; Sakai, Y.; Sakai, H.; Ono, K.; Hashimoto, S.; Matsuura, Y.; Morisada, K. Carbon stock in litter, deadwood and soil in Japan’s forest sector and its comparison with carbon stock in agricultural soils. Soil Sci. Plant Nutr. 2010, 56, 19–30. [Google Scholar] [CrossRef]
- Mashimo, Y. Evaluation of forest growth by quantification of environmental factors. JARQ Jpn. Agric. Res. Q. 1978, 12, 232–237. [Google Scholar]
- Katagiri, N.; Miyake, N.; Fujiwara, Y. Distribution and stand structure of natural Japanese Red-Pine (Pinus Densiflora S. et Z.) in Sanbe Forest of Shimane University. Bull. Fac. Agric. 1987, 21, 39–45. [Google Scholar]
- Umezu, K. History and transition of the coastal forest in Japan—Focusing on the Shonai coastal forest. Tree For. Health 2016, 20, 104–111. [Google Scholar] [CrossRef]
- Franklin, J.F.; Maeda, T.; Ohsumi, Y.; Matsui, M.; Yagi, H.; Hawk, G.M. Subalpine Coniferous Forests of Central Honshu, Japan. Ecol. Monogr. 1979, 49, 311–334. [Google Scholar] [CrossRef]
- Takehara, H.; Kubo, T.; Hosokawa, K. Forest soils derived from granite and palaeozoic sedimentary rocks in Kiso region. J. Jpn. For. Soc. 1959, 41, 436–444. [Google Scholar] [CrossRef]
- Takata, K.; Kurinobu, S.; Koizumi, A.; Yasue, K.; Tamai, Y.; Kisanuki, M. Bibliography on Japanese larch (Larix kaempferi (Lamb.) Carr.). Eurasian J. For. Res. 2005, 8, 111–126. [Google Scholar]
- Haruki, M. Studies on the material biomass of Abies sachalinensis artificial forest. Res. Bull. Hokkai-Do Univ. For. 1979, 36, 147–254. [Google Scholar]
- Yamada, H.; Miyaura, T. Geographic variation in nut size of Castanopsis species in Japan. Ecol. Res. 2004, 20, 3–9. [Google Scholar] [CrossRef]
- Sakai, M.; Inoue, K. Amount of migrated coarse organic matter into soil (V)—Monthly changes in Japanese cedar fallen litter on the forest floor by photograph. In Annual Report of the Shikoku Branch; Forestry and Forest Products Research Institute: Ibaraki, Japan, 1987; Volume 28, pp. 24–27. [Google Scholar]
- Miura, S.; Ugawa, S.; Yoshinaga, S.; Hirai, T.Y.K. Floor Cover Percentage Determines Splash Erosion in Chamaecyparis obtuse Forests. Soil Sci. Soc. Am. J. 2015, 79, 1782–1791. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, T.; Takahashi, T.; Asano, Y. Comparison of Changes in Organic Matter Dynamics due to Stand Age between Artificial Japanese Cedar (Cryptomeria japonica D. Don) Forests and Japanese Cypress (Chamaecyparis obtusa Sieb. et Zucc.) Forests. J. Jpn. For. Soc. 2006, 88, 525–533. [Google Scholar] [CrossRef] [Green Version]
- Kiyono, Y. Analyses of factors affecting Ao-Layer overage in Chamaecypayis obtusa plantations. J. Jpn. For. Soc. 1988, 70, 71–74. [Google Scholar] [CrossRef]
- Miura, S.; Yoshinaga, S.; Yamada, T. Protective effect of floor cover against soil erosion on steep slopes forested with Chamaecyparis obtusa (hinoki) and other species. J. For. Res. 2003, 8, 27–35. [Google Scholar] [CrossRef]
- Hattori, S.; Abe, T.; Kobayashi, C.; Tamai, K. Effect of forest floor coverage on reduction of soil erosion in Hinoki plantations. Bull. For. For. Prod. Res. Inst. 1992, 362, 1–34. [Google Scholar]
- Ichikawa, T.; Takahashi, T.; Kobayashi, T. The relation between the population of the earthworm and types of vegetation or soil environment. J. Jpn. Soc. Reveg. Technol. 2008, 34, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Ohta, T.; Niwa, S.; Agetsuma, N.; Hiura, T. Calcium concentration in leaf litter alters the community composition of soil invertebrates in warm-temperate forests. Pedobiologia 2014, 57, 257–262. [Google Scholar] [CrossRef] [Green Version]
- Takeda, H. A 5-year study of litter decomposition processes in a Chamaecyparis obtusa Endl. forest. Ecol. Res. 1995, 10, 95–104. [Google Scholar] [CrossRef]
- Akai, T.; Asada, S. Studies on natural reproduction (I) The reproduction of Chamaecyparis at Wet Podzolic zone in Kiso district. Bull. Kyoto Univ. For. 1967, 39, 35–63. [Google Scholar]
- Watanabe, M.; Yamaguchi, M.; Tabe, C.; Iwasaki, M.; Yamashita, R.; Funada, R.; Fukami, M.; Matsumura, H.; Kohno, Y.; Izuta, T. Influences of nitrogen load on the growth and photosynthetic responses of Quercus serrata seedlings to O3. Trees 2007, 21, 421–432. [Google Scholar] [CrossRef]
- Yamanaka, T.; Hirai, K.; Aizawa, S.; Yoshinaga, S.; Takahashi, M. Nitrogen-fixing activity in decomposing litter of three tree species at a watershed in eastern Japan. J. For. Res. 2011, 16, 1–7. [Google Scholar] [CrossRef]
- Sawata, S.; Kato, H. Effect of Forest on Soil (Part 2): The base accumulation and other soil properties related to age of Cryptomeria and Japanese cypress stands. Jpn. J. Soil Sci. Plant Nutr. 1991, 62, 49–58. [Google Scholar] [CrossRef]
- Baba, M.; Kato, M.; Sugiura, T.; Kobayashi, H. Calcium accumulation alleviates soil acidification in Japanese cedar (Cryptomeria japonica) stands. Soil Sci. Plant Nutr. 2004, 50, 403–411. [Google Scholar] [CrossRef]
- Tanikawa, T.; Ito, Y.; Fukushima, S.; Yamashita, M.; Sugiyama, A.; Mizoguchi, T.; Okamoto, T.; Hirano, Y. Calcium is cycled tightly in Cryptomeria japonica stands on soils with low acid buffering capacity. For. Ecol. Manag. 2017, 399, 64–73. [Google Scholar] [CrossRef]
- Harada, H.; Satoo, H.; Hotta, I.; Hatiya, K.; Tadaki, Y. Study on the nutrient contents of mature Cryptomeria forest. Bull. Gov. For. Exp. Stn. 1972, 249, 17–74. [Google Scholar]
- Takahashi, M. Water soluble elements in decomposing Japanese cedar needle litter. Soil Sci. Plant Nutr. 1996, 42, 395–399. [Google Scholar] [CrossRef]
- Velázquez, E.; Silva, L.R.; Ramírez-Bahena, M.H.; Peix, A. Diversity of potassium-solubilizing microorganisms and their in-teractions with plants. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: New Delhi, India, 2016; pp. 99–110. ISBN 9788132227762. [Google Scholar]
- Osono, T.; Takeda, H. Potassium, calcium, and magnesium dynamics during litter decomposition in a cool temperate forest. J. For. Res. 2004, 9, 23–31. [Google Scholar] [CrossRef]
- Osono, T. Hyphal length in the forest floor and soil of subtropical, temperate, and subalpine forests. J. For. Res. 2015, 20, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Vinichuk, M.; Taylor, A.; Rosén, K.; Johanson, K. Accumulation of potassium, rubidium and caesium (133Cs and 137Cs) in various fractions of soil and fungi in a Swedish forest. Sci. Total Environ. 2010, 408, 2543–2548. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Muramatsu, Y. Concentrations of radiocesium and potassium in Japanese mushrooms. Environ. Sci. 1994, 7, 63–70. [Google Scholar] [CrossRef]
- Cromack, K.; Todd, R.L.; Monk, C.D. Patterns of basidiomycete nutrient accumulation in conifer and deciduous forest litter. Soil Biol. Biochem. 1975, 7, 265–268. [Google Scholar] [CrossRef]
- Saito, H. Materials for the studies of litterfall in forest stands. Bull. Kyoto Prefect. Univ. For. 1981, 25, 78–89. [Google Scholar]
- Lang, F.; Krüger, J.; Amelung, W.; Willbold, S.; Frossard, E.; Bünemann, E.K.; Bauhus, J.; Nitschke, R.; Kandeler, E.; Marhan, S.; et al. Soil phosphorus supply controls P nutrition strategies of beech forest ecosystems in Central Europe. Biogeochemistry 2017, 136, 5–29. [Google Scholar] [CrossRef] [Green Version]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Yang, Y.; Han, W.; Fang, J. Global patterns of soil microbial nitrogen and phosphorus stoichiometry in forest ecosystems. Glob. Ecol. Biogeogr. 2014, 23, 979–987. [Google Scholar] [CrossRef]
- Kunito, T.; Tsunekawa, M.; Yoshida, S.; Park, H.-D.; Toda, H.; Nagaoka, K.; Saeki, K. Soil Properties Affecting Phosphorus Forms and Phosphatase Activities in Japanese Forest Soils. Soil Sci. 2012, 177, 39–46. [Google Scholar] [CrossRef]
- Nanzyo, M.; Dahlgren, R.; Shoji, S. Chapter 6 Chemical Characteristics of Volcanic Ash Soils. Dev. Soil Sci. 1993, 145–187. [Google Scholar] [CrossRef]
- Stahr, S.; Graf-Rosenfellner, M.; Klysubun, W.; Mikutta, R.; Prietzel, J.; Lang, F. Phosphorus speciation and C:N:P stoichiometry of functional organic matter fractions in temperate forest soils. Plant Soil 2017, 427, 53–69. [Google Scholar] [CrossRef]
- Walker, T.; Syers, J. The fate of phosphorus during pedogenesis. Geoderma 1976, 15, 1–19. [Google Scholar] [CrossRef]
- Johnson, D.W.; Richter, D.D.; Van Miegroet, H.; Cole, D.W. Contributions of Acid Deposition and Natural Processes to Cation Leaching from Forest Soils: A Review. J. Air Pollut. Control. Assoc. 1983, 33, 1036–1041. [Google Scholar] [CrossRef] [Green Version]
- Dijkstra, F.A.; Geibe, C.; Holmström, S.; Lundström, U.S.; Van Breemen, N. The effect of organic acids on base cation leaching from the forest floor under six North American tree species. Eur. J. Soil Sci. 2001, 52, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Vesterdal, L.; Raulund-Rasmussen, K. Forest floor chemistry under seven tree species along a soil fertility gradient. Can. J. For. Res. 1998, 28, 1636–1647. [Google Scholar] [CrossRef]
- Hamburg, S.P.; Yanai, R.D.; Arthur, M.A.; Blum, J.D.; Siccama, T.G. Biotic Control of Calcium Cycling in Northern Hardwood Forests: Acid Rain and Aging Forests. Ecosystems 2003, 6, 399–406. [Google Scholar] [CrossRef]
- MacLean, D.A.; Wein, R.W. Litter production and forest floor nutrient dynamics in pine and hardwood stands of New Brunswick, Canada. Ecography 1978, 1, 1–15. [Google Scholar] [CrossRef]
- Currie, W.S.; Aber, J.D.; Driscoll, C.T. Leaching of nutrient cations from the forest floor: Effects of nitrogen saturation in two long-term manipulations. Can. J. For. Res. 1999, 29, 609–620. [Google Scholar] [CrossRef]
- Wessel, W.W.; Tietema, A. Metal distribution across different pools in the organic layer of a forest under acid deposition and its consequences for the metal dynamics. Plant Soil 1995, 171, 341–350. [Google Scholar] [CrossRef] [Green Version]






| Forest Type | Scientific Names of Predominant Species |
|---|---|
| Cedar | Cryptomeria japonica |
| Cypress | Chamaecyparis obtusa |
| Larch | Larix kaempferi |
| Pine | Pinus densiflora, P. thunbergii |
| Todo fir | Abies sachalinensis |
| Subalpine coniferous | Abies veitchii, A. mariesii, A. sachalinensis, Picea jezoensis var. hondoensis, P. jezoensis, P. glehnii, Tsuga diversifolia |
| Deciduous broad-leaved | Fagus spp., Quercus spp., Betula spp., Acer spp., Alnus spp., Carpinus spp., Pterocarya rhoifolia, Aesculus turbinata, Fraxinus spp. |
| Evergreen broad-leaved | Castanopsis spp., Lithocarpus spp., Quercus acuta, Machilus thunbergii, Cinnamomum camphora, Camellia spp. |
| Nutrient | N | P | K | Ca | Mg | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Forest Type | Mean (2) | s.d. | Mean (2) | s.d. | Mean | s.d. | Mean (2) | s.d. | Mean | s.d. |
| Coniferous | ||||||||||
| Cedar | 10.0 d | 2.86 | 0.542 d | 0.265 | 1.24 | 0.870 | 13.9 a | 4.27 | 1.65 | 1.05 |
| Cypress | 10.0 d | 3.03 | 0.552 cd | 0.270 | 1.70 | 1.46 | 7.11 b | 3.72 | 1.37 | 1.11 |
| Larch | 12.5 bcd | 2.55 | n.a (1) | n.a | 1.55 | 0.815 | 7.37 b | 3.34 | 1.64 | 0.64 |
| Pine | 11.1 cd | 2.67 | 0.770 bcd | 0.195 | 1.51 | 0.597 | 6.42 b | 2.53 | 1.84 | 1.58 |
| Todo fir | 13.4 bc | 1.90 | 1.19 ab | 0.446 | 1.73 | 0.360 | 7.96 b | 3.20 | n.a. | n.a. |
| Subalpine coniferous | 14.0 ab | 3.46 | 1.09 a | 0.624 | 1.48 | 0.574 | 7.47 b | 4.27 | n.a. | n.a. |
| Broad-leaved | ||||||||||
| Deciduous | 15.5 a | 4.83 | 0.813 bc | 0.340 | 1.13 | 0.870 | 7.85 b | 6.53 | 1.33 | 0.888 |
| Evergreen | 13.5 abc | 4.48 | 0.633 cd | 0.270 | 1.37 | 0.647 | 13.7 a | 10.9 | 2.16 | 1.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, M. Nutrient Storage and Stoichiometry of the Forest Floor Organic Matter in Japanese Forests. Soil Syst. 2021, 5, 51. https://doi.org/10.3390/soilsystems5030051
Takahashi M. Nutrient Storage and Stoichiometry of the Forest Floor Organic Matter in Japanese Forests. Soil Systems. 2021; 5(3):51. https://doi.org/10.3390/soilsystems5030051
Chicago/Turabian StyleTakahashi, Masamichi. 2021. "Nutrient Storage and Stoichiometry of the Forest Floor Organic Matter in Japanese Forests" Soil Systems 5, no. 3: 51. https://doi.org/10.3390/soilsystems5030051
APA StyleTakahashi, M. (2021). Nutrient Storage and Stoichiometry of the Forest Floor Organic Matter in Japanese Forests. Soil Systems, 5(3), 51. https://doi.org/10.3390/soilsystems5030051