Surfactants for Electrophoretic Deposition of Polyvinylidene Fluoride–Silica Composites
Abstract
:1. Introduction
2. Materials and Methods
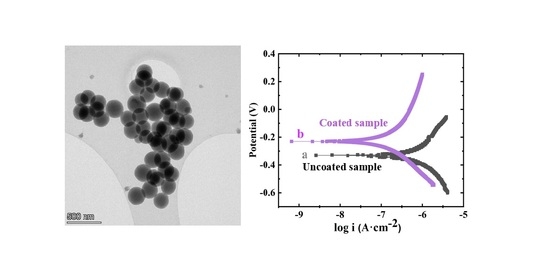
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishyn, V.; Aspermair, P.; Leroux, Y.; Happy, H.; Knoll, W.; Boukherroub, R.; Szunerits, S. “Click” Chemistry on Gold Electrodes Modified with Reduced Graphene Oxide by Electrophoretic Deposition. Surfaces 2019, 2, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Van der Biest, O.O.; Vandeperre, L.J. Electrophoretic deposition of materials. Annu. Rev. Mater. Sci. 1999, 29, 327–352. [Google Scholar] [CrossRef]
- Batool, S.A.; Wadood, A.; Hussain, S.W.; Yasir, M.; Ur Rehman, M.A. A Brief Insight to the Electrophoretic Deposition of PEEK-, Chitosan-, Gelatin-, and Zein-Based Composite Coatings for Biomedical Applications: Recent Developments and Challenges. Surfaces 2021, 4, 205–239. [Google Scholar] [CrossRef]
- Ahmed, Y.; Yasir, M.; Ur Rehman, M.A. Fabrication and Characterization of Zein/Hydroxyapatite Composite Coatings for Biomedical Applications. Surfaces 2020, 3, 237–250. [Google Scholar] [CrossRef]
- Sorkhi, L.; Farrokhi-Rad, M.; Shahrabi, T. Electrophoretic Deposition of Hydroxyapatite–Chitosan–Titania on Stainless Steel 316 L. Surfaces 2019, 2, 458–467. [Google Scholar] [CrossRef] [Green Version]
- Sikkema, R.; Baker, K.; Zhitomirsky, I. Electrophoretic deposition of polymers and proteins for biomedical applications. Adv. Colloid Interface Sci. 2020, 284, 102272. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, R.; Wang, J. Effect of voltage on the mechanical and water resistance properties of zein films by electrophoretic deposition. Food Bioprocess Technol. 2015, 8, 486–491. [Google Scholar] [CrossRef]
- Choudhary, B.; Anwar, S.; Besra, L.; Anwar, S. Electrophoretic deposition studies of Ba (Zr-Ce-Y) O3 ceramic coating. Int. J. Appl. Ceram. Technol. 2019, 16, 1022–1031. [Google Scholar] [CrossRef]
- Dhand, C.; Singh, S.; Arya, S.K.; Datta, M.; Malhotra, B. Cholesterol biosensor based on electrophoretically deposited conducting polymer film derived from nano-structured polyaniline colloidal suspension. Anal. Chim. Acta 2007, 602, 244–251. [Google Scholar] [CrossRef]
- Dange-Delbaere, C.; Buron, C.; Euvrard, M.; Filiâtre, C. Stability and cathodic electrophoretic deposition of polystyrene particles pre-coated with chitosan–alginate multilayer. Colloids Surf. A Physicochem. Eng. Asp. 2016, 493, 1–8. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Verweij, H. Theory of cast formation in electrophoretic deposition. J. Am. Ceram. Soc. 1999, 82, 1451–1455. [Google Scholar] [CrossRef]
- De Riccardis, M.F.; Martina, V.; Carbone, D. Study of polymer particles suspensions for electrophoretic deposition. J. Phys. Chem. B 2013, 117, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Djošić, M.; Mišković-Stanković, V.B.; Kačarević-Popović, Z.M.; Jokić, B.M.; Bibić, N.; Mitrić, M.; Milonjić, S.K.; Jančić-Heinemann, R.; Stojanović, J. Electrochemical synthesis of nanosized monetite powder and its electrophoretic deposition on titanium. Colloids Surf. A Physicochem. Eng. Asp. 2009, 341, 110–117. [Google Scholar] [CrossRef]
- Zhitomirsky, I.; Petric, A. Electrochemical deposition of yttrium oxide. J. Mater. Chem. 2000, 10, 1215–1218. [Google Scholar] [CrossRef]
- Pang, X.; Zhitomirsky, I.; Niewczas, M. Cathodic electrolytic deposition of zirconia films. Surf. Coat. Technol. 2005, 195, 138–146. [Google Scholar] [CrossRef]
- Ruwoldt, J. A Critical Review of the Physicochemical Properties of Lignosulfonates: Chemical Structure and Behavior in Aqueous Solution, at Surfaces and Interfaces. Surfaces 2020, 3, 622–648. [Google Scholar] [CrossRef]
- Ata, M.; Liu, Y.; Zhitomirsky, I. A review of new methods of surface chemical modification, dispersion and electrophoretic deposition of metal oxide particles. RSC Adv. 2014, 4, 22716–22732. [Google Scholar] [CrossRef]
- Ata, M.S.; Poon, R.; Syed, A.M.; Milne, J.; Zhitomirsky, I. New developments in non-covalent surface modification, dispersion and electrophoretic deposition of carbon nanotubes. Carbon 2018, 130, 584–598. [Google Scholar] [CrossRef]
- Biswas, M.; Raichur, A.M. Electrokinetic and rheological properties of nano zirconia in the presence of rhamnolipid biosurfactant. J. Am. Ceram. Soc. 2008, 91, 3197–3201. [Google Scholar] [CrossRef]
- Alves, A.V.; Tsianou, M.; Alexandridis, P. Fluorinated Surfactant Adsorption on Mineral Surfaces: Implications for PFAS Fate and Transport in the Environment. Surfaces 2020, 3, 516–566. [Google Scholar] [CrossRef]
- Nawwar, M.; Poon, R.; Chen, R.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. High areal capacitance of Fe3O4-decorated carbon nanotubes for supercapacitor electrodes. Carbon Energy 2019, 1, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Zhitomirsky, I. Electrophoretic deposition of graphene, carbon nanotubes and composite films using methyl violet dye as a dispersing agent. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 97–103. [Google Scholar] [CrossRef]
- Li, J.; Zhitomirsky, I. Cathodic electrophoretic deposition of manganese dioxide films. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 248–253. [Google Scholar] [CrossRef]
- Ghazali, N.; Basirun, W.J.; Mohammed Nor, A.; Johan, M.R. Super-amphiphobic coating system incorporating functionalized nano-Al2O3 in polyvinylidene fluoride (PVDF) with enhanced corrosion resistance. Coatings 2020, 10, 387. [Google Scholar] [CrossRef] [Green Version]
- Pornea, A.M.; Puguan, J.M.C.; Deonikar, V.G.; Kim, H. Fabrication of multifunctional wax infused porous PVDF film with switchable temperature response surface and anti corrosion property. J. Ind. Eng. Chem. 2020, 82, 211–219. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kwon, Y.S.; Shon, M.Y.; Moon, M.J. Corrosion protection performance of PVDF/PMMA-blended coatings by electrochemical impedance method. J. Electrochem. Sci. Technol. 2018, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, C.; Costa, C.M.; Correia, D.M.; Nunes-Pereira, J.; Oliveira, J.; Martins, P.; Goncalves, R.; Cardoso, V.F.; Lanceros-Mendez, S. Electroactive poly (vinylidene fluoride)-based structures for advanced applications. Nat. Protoc. 2018, 13, 681. [Google Scholar] [CrossRef]
- Ji-Hun, B.; Seung-Hwan, C. PVDF-based ferroelectric polymers and dielectric elastomers for sensor and actuator applications: A review. Funct. Compos. Struct. 2019, 1, 012003. [Google Scholar]
- Inderherbergh, J. Polyvinylidene fluoride (PVDF) appearance, general properties and processing. Ferroelectrics 1991, 115, 295–302. [Google Scholar] [CrossRef]
- Zhong, J.; Li, W.; Qian, J.; Fu, C.; Chu, H.; Xu, J.; Ran, X.; Nie, W. Modulation of the interfacial architecture enhancing the efficiency and energy density of ferroelectric nanocomposites via the irradiation method. J. Colloid Interface Sci. 2021, 586, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Hernandez, C.; Zuniga, J.P.; Lozano, K.; Mao, Y. Luminescent PVDF nanocomposite films and fibers encapsulated with La 2 Hf 2 O 7: Eu 3+ nanoparticles. SN Appl. Sci. 2020, 2, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhu, X.; Zhang, T.; Bano, S.; Pan, H.; Qi, L.; Zhang, Z.; Yuan, Y. A renewable low-frequency acoustic energy harvesting noise barrier for high-speed railways using a Helmholtz resonator and a PVDF film. Appl. Energy 2018, 230, 52–61. [Google Scholar] [CrossRef]
- Hu, P.; Yan, L.; Zhao, C.; Zhang, Y.; Niu, J. Double-layer structured PVDF nanocomposite film designed for flexible nanogenerator exhibiting enhanced piezoelectric output and mechanical property. Compos. Sci. Technol. 2018, 168, 327–335. [Google Scholar] [CrossRef]
- Park, J.H.; Kurra, N.; AlMadhoun, M.; Odeh, I.N.; Alshareef, H.N. A two-step annealing process for enhancing the ferroelectric properties of poly (vinylidene fluoride)(PVDF) devices. J. Mater. Chem. C 2015, 3, 2366–2370. [Google Scholar] [CrossRef]
- Foster, F.S.; Harasiewicz, K.A.; Sherar, M.D. A history of medical and biological imaging with polyvinylidene fluoride (PVDF) transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2000, 47, 1363–1371. [Google Scholar] [CrossRef]
- Kang, G.-d.; Cao, Y.-m. Application and modification of poly (vinylidene fluoride)(PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Correia, D.M.; Gonçalves, R.; de Zea Bermudez, V.; Silva, M.M.; Lanceros-Mendez, S.; Costa, C.M. Enhanced ionic conductivity in poly(vinylidene fluoride) electrospun separator membranes blended with different ionic liquids for lithium ion batteries. J. Colloid Interface Sci. 2021, 582, 376–386. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, L.; Zheng, J.; Wang, Z.; Li, B.; Yan, Y.; Meng, M. Synergistic interaction of Z-scheme 2D/3D g-C3N4/BiOI heterojunction and porous PVDF membrane for greatly improving the photodegradation efficiency of tetracycline. J. Colloid Interface Sci. 2021, 586, 335–348. [Google Scholar] [CrossRef]
- Wei, N.; Li, Z.; Li, Q.; Yang, E.; Xu, R.; Song, X.; Sun, J.; Dou, C.; Tian, J.; Cui, H. Scalable and low-cost fabrication of hydrophobic PVDF/WS2 porous membrane for highly efficient solar steam generation. J. Colloid Interface Sci. 2021, 588, 369–377. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, J.; Ren, W.; Xu, F.; Wu, X.; Shi, P.; Ye, Z.-G. A new biosensor based on PVDF film for detection of nucleic acids. Ceram. Int. 2015, 41, S602–S606. [Google Scholar] [CrossRef]
- Song, Y.-S.; Yun, Y.; Lee, D.Y.; Kim, B.-Y. Effect of PVDF Concentration and Number of Fiber Lines on Piezoelectric Properties of Polymeric PVDF Biosensors. Fibers Polym. 2021, 22, 1200–1207. [Google Scholar] [CrossRef]
- Hartono, A.; Sanjaya, E.; Ramli, R. Glucose sensing using capacitive biosensor based on polyvinylidene fluoride thin film. Biosensors 2018, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Häsler, E.; Stein, L.; Harbauer, G. Implantable physiological power supply with PVDF film. Ferroelectrics 1984, 60, 277–282. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, H.; Orbay, H.; Chen, F.; England, C.G.; Cai, W.; Wang, X. Biocompatibility and in vivo operation of implantable mesoporous PVDF-based nanogenerators. Nano Energy 2016, 27, 275–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.; Ding, W.; Liu, J.; Yang, B. Flexible PVDF based piezoelectric nanogenerators. Nano Energy 2020, 78, 105251. [Google Scholar] [CrossRef]
- Houis, S.; Engelhardt, E.; Wurm, F.; Gries, T. Application of polyvinylidene fluoride (PVDF) as a biomaterial in medical textiles. In Medical and Healthcare Textiles; Elsevier: Amsterdam, The Netherlands, 2010; pp. 342–352. [Google Scholar]
- Wang, H.; Klosterhalfen, B.; Müllen, A.; Otto, T.; Dievernich, A.; Jockenhövel, S. Degradation resistance of PVDF mesh in vivo in comparison to PP mesh. J. Mech. Behav. Biomed. Mater. 2021, 119, 104490. [Google Scholar] [CrossRef]
- Haddadi, S.A.; Ghaderi, S.; Amini, M.; Ramazani, S.A. Mechanical and piezoelectric characterizations of electrospun PVDF-nanosilica fibrous scaffolds for biomedical applications. Mater. Today Proc. 2018, 5, 15710–15716. [Google Scholar] [CrossRef]
- Kyeremateng, N.A.; Gukte, D.; Ferch, M.; Buk, J.; Hrebicek, T.; Hahn, R. Preparation of a Self-Supported SiO2 Membrane as a Separator for Lithium-Ion Batteries. Batter. Supercaps 2020, 3, 456–462. [Google Scholar] [CrossRef]
- Prasanna, K.; Subburaj, T.; Jo, Y.N.; Lee, C.W. Optimization of electrophoretic suspension to fabricate Li[Ni1/3Co1/3Mn1/3]O2 based positive electrode for Li-ion batteries. Electrochim. Acta 2013, 95, 295–300. [Google Scholar] [CrossRef]
- Hagberg, J.; Maples, H.A.; Alvim, K.S.; Xu, J.; Johannisson, W.; Bismarck, A.; Zenkert, D.; Lindbergh, G. Lithium iron phosphate coated carbon fiber electrodes for structural lithium ion batteries. Compos. Sci. Technol. 2018, 162, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Ui, K.; OKURA, K.; Koura, N.; Tsumeda, S.; Tamamitsu, K. Fabrication of the electrode for capacitor cell prepared by the electrophoretic deposition method. Electrochemistry 2007, 75, 604–606. [Google Scholar] [CrossRef] [Green Version]
- Lau, K.T.; Suan, M.S.M.; Zaimi, M.; Abd Razak, J.; Azam, M.; Mohamad, N. Microstructure and Phase of Poly (Vinyliden Fluoride) Films by Electrophoretic Deposition: Effect of Polymer Dispersion’s Stirring Conditions. J. Adv. Manuf. Technol. (JAMT) 2016, 10, 57–66. [Google Scholar]
- Lau, K.T.; Ab Razak, M.H.R.; Kok, S.L.; Zaimi, M.; Abd Rashid, M.W.; Mohamad, N.; Azam, M.A. Electrophoretic Deposition and Heat Treatment of Steel-Supported PVDF-Graphite Composite Film. In Applied Mechanics and Materials; Trans Tech Publications: Zurich, Switzerland, 2015; pp. 412–416. [Google Scholar]
- Yin, J.; Fukui, T.; Murata, K.; Matsuda, M.; Miyake, M.; Hirabayashi, T.; Yamamuro, S. Fabrication of protective KB/PVdF composite films on stainless steel substrates for PEFCs through electrophoretic deposition. J. Ceram. Soc. Jpn. 2008, 116, 201–204. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Veldhuis, S.; Mathews, R.; Zhitomirsky, I. Influence of chemical structure of bile acid dispersants on electrophoretic deposition of poly (vinylidene fluoride) and composites. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127181. [Google Scholar] [CrossRef]
- Hashiba, M.; Okamoto, H.; Nurishi, Y.; Hiramatsu, K. The zeta-potential measurement for concentrated aqueous suspension by improved electrophoretic mass transport apparatus—application to Al2O3, ZrO3 and SiC suspensions. J. Mater. Sci. 1988, 23, 2893–2896. [Google Scholar] [CrossRef]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-inspired adhesives and coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 2007, 448, 338–341. [Google Scholar] [CrossRef]
- Xu, N.; Li, Y.; Zheng, T.; Xiao, L.; Liu, Y.; Chen, S.; Zhang, D. A mussel-inspired strategy for CNT/carbon fiber reinforced epoxy composite by hierarchical surface modification. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 128085. [Google Scholar] [CrossRef]
- Sun, Y.; Ata, M.; Zhitomirsky, I. Electrophoretic deposition of TiO2 nanoparticles using organic dyes. J. Colloid Interface Sci. 2012, 369, 395–401. [Google Scholar] [CrossRef]
- Sun, Y.; Zhitomirsky, I. Electrophoretic deposition of titanium dioxide using organic acids as charging additives. Mater. Lett. 2012, 73, 190–193. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Zhitomirsky, I. Dispersing agents for electrophoretic deposition of TiO2 and TiO2–carbon nanotube composites. Colloids Surf. A Physicochem. Eng. Asp. 2013, 418, 131–138. [Google Scholar] [CrossRef]
- Ata, M.; Zhitomirsky, I. Preparation of MnO2 and composites for ultracapacitors. Mater. Manuf. Processes 2013, 28, 1014–1018. [Google Scholar]
- Wu, K.; Wang, Y.; Zhitomirsky, I. Electrophoretic deposition of TiO2 and composite TiO2–MnO2 films using benzoic acid and phenolic molecules as charging additives. J. Colloid Interface Sci. 2010, 352, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhitomirsky, I. Bio-inspired catechol chemistry for electrophoretic nanotechnology of oxide films. J. Colloid Interface Sci. 2012, 380, 8–15. [Google Scholar] [CrossRef]
- Sharma, T.; Je, S.-S.; Gill, B.; Zhang, J.X. Patterning piezoelectric thin film PVDF–TrFE based pressure sensor for catheter application. Sens. Actuators A Phys. 2012, 177, 87–92. [Google Scholar] [CrossRef]
- Bhatt, A.S.; Bhat, D.K.; Santosh, M. Crystallinity, conductivity, and magnetic properties of PVDF-Fe3O4 composite films. J. Appl. Polym. Sci. 2011, 119, 968–972. [Google Scholar] [CrossRef]
- Venevtsev, Y.N.; Gagulin, V.V.; Zhitomirsky, I.D. Material science aspects of seignette-magnetism problem. Ferroelectrics 1987, 73, 221–248. [Google Scholar] [CrossRef]
- Luo, H.; Hanagud, S. PVDF film sensor and its applications in damage detection. J. Aerosp. Eng. 1999, 12, 23–30. [Google Scholar] [CrossRef]
- Li, W.; Song, Z.; Zhong, J.; Qian, J.; Tan, Z.; Wu, X.; Chu, H.; Nie, W.; Ran, X. Multilayer-structured transparent MXene/PVDF film with excellent dielectric and energy storage performance. J. Mater. Chem. C 2019, 7, 10371–10378. [Google Scholar] [CrossRef]
- Kobayashi, M.; Tashiro, K.; Tadokoro, H. Molecular vibrations of three crystal forms of poly (vinylidene fluoride). Macromolecules 1975, 8, 158–171. [Google Scholar] [CrossRef]
- Zeng, Z.; Yu, D.; He, Z.; Liu, J.; Xiao, F.-X.; Zhang, Y.; Wang, R.; Bhattacharyya, D.; Tan, T.T.Y. Graphene oxide quantum dots covalently functionalized PVDF membrane with significantly-enhanced bactericidal and antibiofouling performances. Sci. Rep. 2016, 6, 20142. [Google Scholar] [CrossRef] [PubMed] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhitomirsky, I. Surfactants for Electrophoretic Deposition of Polyvinylidene Fluoride–Silica Composites. Surfaces 2022, 5, 308-317. https://doi.org/10.3390/surfaces5020022
Wang Z, Zhitomirsky I. Surfactants for Electrophoretic Deposition of Polyvinylidene Fluoride–Silica Composites. Surfaces. 2022; 5(2):308-317. https://doi.org/10.3390/surfaces5020022
Chicago/Turabian StyleWang, Zhengzheng, and Igor Zhitomirsky. 2022. "Surfactants for Electrophoretic Deposition of Polyvinylidene Fluoride–Silica Composites" Surfaces 5, no. 2: 308-317. https://doi.org/10.3390/surfaces5020022
APA StyleWang, Z., & Zhitomirsky, I. (2022). Surfactants for Electrophoretic Deposition of Polyvinylidene Fluoride–Silica Composites. Surfaces, 5(2), 308-317. https://doi.org/10.3390/surfaces5020022