3.1. Catalyst Characterization
The elemental analysis confirms the absence of C, S, and N on the parent SBA-15 material, and the presence of these elements on IL-HSO
4-SBA-15, and IL-Cl-SBA-15 at levels close to those theoretically expected (see
Table 1). The level of S on the IL-HSO
4-SBA-15 material is almost twice that on the IL-Cl-SBA-15 material (reflecting the S atoms also present in the former material’s counter-ions).
Figure 2 shows (a) displaced BET adsorption/desorption isotherms for SBA-15 (red), IL-Cl-SBA-15 (orange), IL-HSO
4-SBA-15 (purple), and (b) the corresponding pore size distribution profiles obtained by applying the BJH method to the desorption branch of the isotherm (the numbers beside the peaks indicate the pore diameter at the maximum point).
Table 1 shows the corresponding BET surface areas, pore sizes, and volumes.
The parent SBA-15 material, as expected, has a surface area of 552 m
2 g
−1 and the desorption isotherm shows a H1 hysteresis loop. Functionalization of the support with the components of the HSO
4− and Cl
−-containing ionic liquids still yielded materials that gave the Type IV isotherm with H1 hysteresis loop to be maintained. However, there was a decrease in the surface areas upon functionalization. The pore volume also decreased, while the pore diameter increased. The values obtained are still in keeping with literature values [
16].
There is a small broad peak in the pore size distribution profile of IL-HSO4-SBA-15, ranging between 24–84 nm, this leads to the higher average pore size measured.
TEM images of the parent SBA-15 material and the two IL-modified analogues are shown in
Figure 3.
Figure 3a,b shows a typical TEM image of SBA-15 taken perpendicular to the pore channels and confirms the characteristic long-range open pore channels that are associated with its framework. This characteristic feature is typical for a mesostructure with 2D p6mm hexagonal symmetry [
24,
25].
Figure 3b is taken parallel to the pore channels and shows the hexagonal pores associated with SBA-15. The average channel size of SBA-15 was found to be 3.2 ± 0.6 nm (
n = 30), which is in keeping with literature values [
20,
26], but found to be somewhat lower than values obtained by treatment of N
2 physisorption isotherm data (4.3 nm).
The discrepancies between the TEM channel (pore) sizes and BJH pore sizes is unexpected as BJH is generally thought to underestimate the pore sizes of materials [
27]. It is thought that the discrepancies between the two arise from the fact that only the pore channel is measured using TEM, whereas N
2 physisorption measurements measure every pore (including macros-pores between SiO
2 particles) which contributes to a larger average pore size.
Following the tethering of ionic liquid components (
Figure 3c,d), it is possible to see that the long-range order, open pore channels in (a) (IL-HSO
4) and the hexagonal pore shape in (b) (IL-Cl) remain intact.
The average channel size of the IL materials are 2.9 ± 0.5 nm (n = 50) for IL-HSO4-SBA-15 and 3.1 ± 0.4 nm (n = 50) for IL-Cl-SBA-15. These values are somewhat lower than for the parent material, showing that tethering of the synthesized ILs does seem to decrease the channel or pore size but does not affect the overall structural integrity of the support.
Thermogravimetry was used to confirm the tethering of the IL to the surface of the SBA. TGA of SBA-15 showed the expected profile for a pre-calcined sample, i.e., a mass loss at low temperatures (~100 °C) as adsorbed H2O was removed from the surface and then a smaller mass loss at higher temperatures as surface hydroxyl condensation (to form removable water) took place. Mass spectrometer profiles confirmed H2O evolution while no CO2 was noted in the exit gas. Total mass loss following treatment to 800 °C was ~1.5%.
Figure 4 shows the TGA and DTGA profiles when the HSO
4—containing ionic liquid and the IL-HSO
4-SBA-15 catalyst were analyzed by thermogravimetry (along with the associated mass spectrometer profiles of selected exit gases).
The pure IL HSO4 sample completely combusted in three major events (peaking at ~200, 350, and 550 °C) with concomitant evolution of CO2, NO2, SO2, H2O, and propyl fragments. No evidence of Si species in the gas phase (or remaining on the weighing pan following the experiment) was noted, and this suggests the formation and removal of an undetected gaseous species (possibly a silicon alkoxide).
Once tethered to SBA-15, the weight loss from the IL-HSO
4-SBA-15 composite material following TGA was ~26% (see
Figure 4c) and again CO
2, H
2O, and SO
2 are noted in the exhaust gas. The evolution of NO
2 (which arises from the combustion of the imidazolium ring) is not noted, and it is possible that this is below the limit of detection of the mass spectrometer. The mass losses (when water desorption is ignored) confirm the expected loadings of the HSO
4 IL onto the SBA-15 material.
The relative importance of the three combustion events noted when the IL combusts prior to tethering are changed following tethering, and the material appears to be more resistant to oxidation–with the principal peak maximum moving from ~350 to ~400 °C.
Figure 5 shows the equivalent profiles from the Cl-containing IL. In general, higher temperatures are required to oxidize this material—with DTGA peaks at 250, 400, and 600 °C. This suggests that the Cl
− counter ion has a stabilizing effect. The pure sample (when combusted to 900 °C) leaves a white residue (of ~16% of the initial IL mass) and the FTIR spectrum of the residue suggests the formation of a siliconoxychloride species [
28,
29].
During the temperature ramp, mass spectrometer profiles relating to CO2, H2O, NO2, alkyl fragments, SO2, and HCl are noted at temperatures co-incident with the DTGA mass loss peaks.
As was seen in the case of IL-HSO
4-SBA-15, tethering IL-Cl to SBA-15 changed the decomposition/oxidation temperatures of the components of the IL (see
Figure 5c,d), with the highest decomposition temperature event now peaking at 550 °C and the lower and higher temperatures peaks reduced in size (comparing (a) to (c)).
As was also the case in the MS analysis of the IL-HSO4-SBA-15 sample, NO2 was not detected evolving from IL-Cl-SBA-15, again suggesting that the amounts of NO2 generated are below the limits of detection of the mass spectrometer. A similar situation relates to attempts to monitor HCl formation during this experiment (i.e., it was visible during oxidation of the pure IL, but not during the oxidation of the tethered species).
Again, the overall mass loss (~20% in this case) is in the same range as would be expected given the amount of IL coordinated to the surface.
In conclusion, the TGA results show that both ILs are tethered to the SBA-15 surface and also show that both the tethering and the different counter-ions affect the stability of the cationic component.
Figure 6 shows the FTIR spectrum of the two ionic liquids, Il-HSO
4 (blue) and IL-Cl (orange). The ILs cation structure is shown as an inset.
The peaks found at 3152 and between 3000–2961 cm
−1 are associated with C-H stretches of both the imidazolium ring and the alkyl chain, respectively. Another C-H stretch is found at 1457 cm
−1 and is due to deformation vibrations of the alkyl chain. At 1589 cm
−1 the stretching vibration of C = N can be observed, and this corresponds to C-N bonds within the imidazolium ring. Finally, peaks located between 1110–990 cm
−1 are those of the Si-O-CH
3 stretching vibrations. Peaks that attributed to sulfonic acid S = O and were relevant HSO
4− are within this region [
16] and cannot be seen due to the overlap with bands relating to the Si-O-CH
3 peaks.
In addition, of note in the IL-Cl spectrum is the presence of an OH band between 3600–3300 cm
−1. This feature could be due to hydrogen bonding of water molecules to the IL, or possibly due to Si-OH groups formed following hydrolysis of Si-O-CH
3 [
30].
Figure 7 shows the FTIR spectra of SBA-15 (red), IL-HSO
4-SBA-15 (green), and IL-Cl-SBA-15 (purple). When IL is attached to the surface of SBA-15, the peak at 3745 cm
−1 (which relates to vibrations of isolated silanols on the SBA-15 surface) decreases in intensity. This confirms that these species are involved with the condensation reaction with the alkoxy silane groups on the IL cations.
The hydrocarbon stretches of both the imidazolium ring and alkyl chains are present in both the spectra tethered IL spectra, peaking at 3152 cm−1 and between 3000–2961 cm−1, respectively. There is also evidence for the presence of the C = N bond in the spectra of tethered ILs, with a small peak at 1589 cm−1. Stretching vibration peaks of S = O and Si-O-CH3 should be present at 1208 cm−1 and between 1110–990 cm−1 but overlap with peaks associated with the Si-O-Si, occurring between 1200–1350 cm−1 (the peak at 1338 cm−1 is noted in the SBA-15 (red) spectrum for reference).
3.2. Catalyst Acidicy Characterization
In another FTIR characterization, D-FTIR of the materials were collected following their interactions with NH
3(g). In these experiments the catalyst was used as the spectral background, and the spectra are plotted as absorbance vs. cm
−1. Therefore, absorbance in the spectra correspond to features added to the surface following adsorption of NH
3, and peaks pointing downwards correspond to features removed from the surface following its interaction with NH
3. Literature reports [
31,
32] suggest that positive peaks at 1681 and 1450 cm
−1 can be attributed to Brønsted acids, due to the formation of the ammonium ion (NH
4+) while positive peaks found at 1630 cm
−1 are a result of NH
3 coordination to Lewis acid sites.
The difference FTIR spectra of NH
3 adsorbed on SBA-15 (red), IL-Cl-SBA-15 (orange), and IL-HSO
4-SBA-15 (purple) catalysts in the regions of 4000–2000 cm
−1 (a) and 1900–1300 cm
−1 (b) are shown in
Figure 8.
There was no evidence for adsorption of NH3 onto the surface of unmodified SBA-15. The spectra in (a) confirm the coordination of NH3 with surface silanol groups (note the negative band 3600–3400 cm−1) of the modified catalysts.
Both negative and positive peaks can be observed in the 1900–1300 cm−1 spectra (b). The negative peaks found at 1630 (on the Cl-containing catalyst) and 1589 cm−1 (on both catalysts) are due to the disappearance of a surface feature related to surface silanols and NH3 adsorption to the imidazolium (C-N stretching vibration) ring, respectively (confirming adsorption on these functionalities).
Relatively intense bands are observed between 1600–1400 cm−1. These relate to adsorbed NH3 acting as a Brønsted base (i.e., forming NH4+). These bands have an irregular shape suggesting the overlap of several different NH4+ species (e.g., adsorbed on support, tether SO3H species) making deconvolution of the positive and negative peaks in this region difficult.
Another positive peak at 1681 cm−1 is observed in the spectrum of NH3 adsorbed on the IL-Cl-SBA-15 catalyst.
These experiments suggest that (a) the tethered IL are able to adsorb NH3 and (b) there is a difference between how NH3 interacts with IL-HSO4-SBA-15 and how it interacts with IL-Cl-SBA-15 (note two NH4+ peaks on the chloride-containing sample).
The NH
3 temperature programmed desorption was also used to characterize the acidity of the catalysts.
Figure 9 shows the NH
3 TPD profiles from the surface of SBA-15 (red), IL-Cl-SBA-15 (orange), and IL-HSO
4-SBA-15 (purple). These profiles have been manually displaced.
No NH3 desorbed from the SBA-15 material. Conversely, the NH3 profile from IL-Cl-SBA-15 evolved one broad NH3 desorption peak, between 180–560 °C and the catalyst had an NH3 adsorption capacity of 29 μmol NH3 g−1. Given that the desorption peak is so broad it is difficult to quantitatively describe the acid strength of the catalyst, but it is clear that a wide range of strengths are present.
The IL-HSO4-SBA-15 profile had more well-defined desorption peaks, the first between 220–400 °C and the second (and larger) peak between 410–650 °C. It should be recalled that the tethers themselves decompose at these temperatures, and may generate NH3 during this decomposition (although no NH3 was noted during experiments where none had been dosed to either of these catalysts).
This second (higher strength) adsorption site has a higher adsorption capacity than the first adsorption site (64 and 12 μmol g−1 NH3 desorption, respectively). It is probable that the HSO4− counterion associated with this tethered ionic liquid component played a role in the NH3 adsorption. This counterion has effectively doubled the [S] content but should also be of sufficient acid strength to coordinate NH3. It is clear, according to this measurement that IL-HSO4-SBA-15 has a higher concentration of acid sites than IL-Cl-SBA-15.
The S content, and therefore the number of tethered species on IL-Cl-SBA-15 and IL-HSO4-SBA-15, are 925 and 1437 μmol g−1, respectively (based on [S] measured during the elemental analysis). However, the amount of NH3 adsorbed on (and desorbed from) each catalyst during NH3 TPD is far lower, i.e., IL-Cl-SBA-15 and IL-HSO4-SBA-15 desorb 29 and 76 μmol g−1, respectively. This indicates that a significant proportion of tethered acid sites (more than 95% in total, be they SO3H or HSO4−) are unavailable for NH3 adsorption during the adsorption steps of these experiments.
This low adsorption capacity may be related to the interaction of tethered SO3H groups with free surface OH groups. These may then remain ‘flat-lying’ on the surface hydrogen bonding to these surface OH groups (and therefore be unavailable for NH3 adsorption).
Acid capacity measurements were used to determine the number of available H+ g−1 of catalyst and can be used as a comparative measure to the NH3 TPD results.
The catalysts were titrated with NaOH (0.01 M) using phenolphthalein (0.5 wt% in ethanol and water (1:1)) as an indicator with a color change from cloudy white to cloudy pink being associated with the neutralization point [
33,
34].
This shows SBA 15 as having 0.14 mmol H+ g−1, while of the two IL catalysts, IL-Cl-SBA-15 had 0.37 mmol H+ g−1 and IL-HSO4-SBA-15 had the highest concentration of available acid sites, with a concentration of 1.9 mmol g−1.
Finally, regarding characterization of acidity, Hammett indicators [
23] were used to give a range of acid strengths of the materials. Each indicator has a specific pKa–the lower the pKa the stronger the acid. By placing the Hammett indicators on the surface of the catalyst a pKa range for the surface acid strength can be found. If the color is that of the acid form of the indicator, then the value of the H
0 function of the solid is equal to or lower than the pKa of the conjugate acid of the indicator. This experiment confirmed that SBA and IL-Cl-SBA-15 had pKa values between 4.8 and 0.8 (being acidic to methyl red but not to crystal violet indicators), while IL-HSO
4-SBA-15 had a pKa between 0.8 and −3.0 (being acidic to crystal violet, but not to dicinnamalacetone).
These results confirm that, notwithstanding the fact that the SO3H loadings were comparable in both catalysts, the IL-Cl-SBA-15 acid sites were both weaker and less concentrated than those of IL-the HSO4-SBA-15 catalyst. This must relate to an effect of the HSO4− counter ion, possibly acting as an acid site.
3.3. Reactivity Measurements
The reactivity of SBA-15 and both IL catalysts were probed in the VA + V-OH esterification reaction, under the experimental conditions outlined in the experimental section. The reactivity of these heterogeneous catalysts were compared to that of a model homogeneous catalyst (ρ-toluene sulfonic acid) [
35] and reactions in the absence of catalyst. When catalyzed, these reactions were carried out with similar concentrations of SO
3H to allow comparisons of reactivity.
Figure 10a–d shows the removal of VA and the formation of PV in the absence of catalyst and the presence of unmodified SBA-15 (a), in the presence of a homogeneous catalyst p-toluene sulfonic acid (at the same levels as the sulfonic acid on each of the IL-SBA catalysts) (b), in the presence of IL-HSO
4-SBA-15 (c) and IL-Cl-SBA-15 (d).
The un-catalyzed reaction forms as much PV as the reaction in the presence of unmodified SBA-15 (~13% after 5 h reaction) although the latter does remove significantly more VA (through adsorption rather than reaction). This is reflected in the carbon balance of the reaction over unmodified SBA-15 (66% after 5 h).
The reaction in the absence of added catalyst is catalyzed by the VA reactant (which itself can donate a proton to initiate the reaction).
Figure 10b shows the removal of VA and the formation of PV in the reaction in the presence of a homogeneous catalyst (p-toluene sulfonic acid) at -SO
3H loadings equivalent to those used in the tethered IL catalyzed reactions. Over 90% of the VA is converted to PV after 1 h during this reaction (showing how active sulfonic acid catalysts can be).
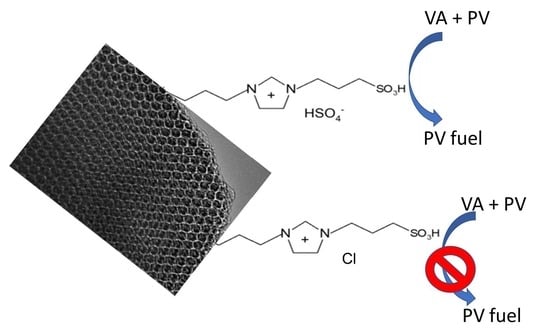
Figure 10c,d shows the conversions of VA and the formation of PV over both of the tethered IL materials and it is clear that, while both are significantly less active than the homogeneous analogue, the IL-HSO
4-SBA-15 catalyst (forming 78% of the possible PV product after 5 h) is far more active than the IL-Cl-SBA-15 material (forming 19% of the possible PV product after 5 h).
The tethered IL catalysts also show far higher carbon balances than the unmodified SBA-15 (90% and 99% compared to 66%). This suggests that surface hydroxyls are the adsorption sites for VA on SBA-15 and their removal (through condensation in the preparation of the tethered IL catalysts) hinders this adsorption.
The reactivity of the IL-Cl-SBA-15 and IL-HSO4-SBA15 catalysts were measured in a recyclability experiment. In these reactions the used catalysts were washed in CH3OH and dried at 80 °C overnight before reuse.
The reactivity over both catalysts was lower than the activity of the analogous fresh catalyst. In the case of the IL-Cl-SBA-15 material, conversion to PV fell to the same level as was seen in the uncatalyzed reaction (or the reaction over SBA-15), while in the case of the IL-HSO4-SBA-15 catalyst, conversion after 5 h conversion to PV fell from 78% to 60%.
The IL-HSO4-SBA-15 catalyst had a higher acidity (both in terms of concentration and acid strength) than IL-Cl-SBA-15 and also had a higher activity in promotion of the desired reaction. The extent of the difference between the reactivity of the two materials is somewhat surprising considering that both catalysts have the same cation and (to a first approximation) the same surface concentration of HSO3.
It is clear that the different counterions have an effect on both the acidity and the activity of the catalyst (and it is assumed that the former influences the latter).
The IL-Cl-SBA-15 catalyst appears to have no significant catalytic effect whereas the catalyst containing the HSO4− counter ion appears to have a substantial beneficial effect on the activity of the catalyst.
It is possible that the HSO
4− ion itself is also acting as a catalyst. This species can potentially donate a proton (initiating the reaction). However, HSO
4− is considered a weak acid with a pKa of +1.9 and a K
a of 1.2 × 10
−2, whereas H
2SO
4, for example, with a pKa of 3.0 and a K
a of 1 × 10
3 [
36]. Given that it is such a weak acid it may simply be coordinating the reactants to the surface of the catalyst, orienting them for reaction as opposed to donating a proton and initiating the reaction.
Post-reaction characterization was also carried out on the three materials.
TGA profiles from post reaction SBA-15 show a mass loss of 17.5% (compared to 1.5% mass loss from the fresh catalyst). The fresh catalyst lost mass during TGA from dehydration and dihydroxylation, while the post reaction catalyst lost mass due to combustion of adsorbed VA from the reaction mixture (D-FTIR analysis of post reaction SBA-15 confirms a carbonyl stretch of adsorbed VA and not PV).
FTIR of the post-reaction IL-HSO4-SBA-15 and IL-Cl-SBA-15 catalysts also shows evidence for VA (and not PV), adsorption on both materials and this relates to the measured carbon balances (which were 90% and 99% over the IL-HSO4-SBA-15 and IL-Cl-SBA-15 catalysts, respectively).
TGA from the post reaction IL-HSO4-SBA-15 and IL-Cl-SBA-15 catalysts (results not shown) show that, notwithstanding the adsorption of some reactants on the catalysts, the mass losses from the post-reaction materials during TGA were lower than those from the fresh IL-HSO4-SBA-15 and IL-Cl-SBA-15 catalysts.
The fresh IL-HSO4-SBA-15 catalyst lost 19.7% during TGA while the post-reaction catalyst lost 17.6% of its mass. The fresh IL-Cl-SBA-15 lost 18.8% of its mass during TGA, while its post reaction analogue lost 14.9% of its mass.
These results suggest that some of the tethered species were removed (possibly hydrolyzed with water produced during the esterification reaction) from the surface during the reactions.