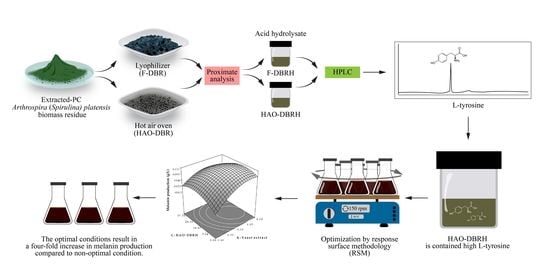
Optimization of Melanin Production by Streptomyces antibioticus NRRL B-1701 Using Arthrospira (Spirulina) platensis Residues Hydrolysates as Low-Cost L-tyrosine Supplement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Dried Biomass Residue and Biomass Residue Composition Analysis by Proximate Analysis
2.2. Acid-Hydrolysis Treatment
2.3. Quantitative Analysis of L-tyrosine by Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC)
2.3.1. Pre-Column Derivatization
2.3.2. RP-HPLC Analysis
2.4. Optimization of the Melanin Production Medium by Response Surface Methodology (RSM) through Central Composite Design (CCD)
2.4.1. Preparation of the Inoculum and Media
2.4.2. CCD and RSM
2.5. Cost Analysis
2.6. Statistical Analysis
3. Results
3.1. Preparation of the Dried Biomass Residue and Analysis of Biomass Residue Composition by Proximate Analysis
3.2. L-tyrosine Quantitative Analysis by Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC)
3.3. Optimization of the Melanin Production Medium by Response Surface Methodology (RSM) through Central Composite Design (CCD)
3.4. Cost Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Simon, J.D. Isolation and biophysical studies of natural eumelanins: Applications of imaging technologies and ultrafast spectroscopy. Pigment Cell Res. 2003, 16, 606–618. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; Saber, W.I. Natural melanin: Current trends, and future approaches, with especial reference to microbial source. Polymers 2022, 14, 1339. [Google Scholar] [CrossRef]
- Solano, F. Melanin and melanin-related polymers as materials with biomedical and biotechnological applications-cuttlefish ink and mussel foot proteins as inspired biomolecules. Int. J. Mol. Sci. 2017, 18, 1561. [Google Scholar] [CrossRef]
- Mostert, A.B. Melanin, the what, the why and the how: An introductory review for materials scientists interested in flexible and versatile polymers. Polymers 2021, 13, 1670. [Google Scholar] [CrossRef]
- Mbonyiryivuze, A.; Mwakikunga, B.W.; Dhlamini, S.M.; Maaza, M. Fourier transform infrared spectroscopy for sepia melanin. Phys. Math. Chem. 2015, 3, 25–29. [Google Scholar]
- Pralea, I.E.; Moldovan, R.C.; Petrache, A.M.; Ilieș, M.; Hegheș, S.C.; Ielciu, I.; Nicoară, R.; Moldovan, M.; Ene, M.; Radu, M.; et al. From extraction to advanced analytical methods: The challenges of melanin analysis. Int. J. Mol. Sci. 2019, 20, 3943. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.Y.; Jang, S.; Sudheer, P.D.; Choi, K.Y. Microbial production of melanin pigments from caffeic acid and L-tyrosine using Streptomyces glaucescens and FCS-ECH-expressing Escherichia coli. Int. J. Mol. Sci. 2021, 22, 2413. [Google Scholar] [CrossRef]
- Barbuto Ferraiuolo, S.; Cammarota, M.; Schiraldi, C.; Restaino, O.F. Streptomycetes as platform for biotechnological production processes of drugs. Appl. Microbiol. Biotechnol. 2021, 105, 551–568. [Google Scholar] [CrossRef]
- Dholakiya, R.N.; Kumar, M.A.; Kalpana, H.M. Production and characterization of melanin from Streptomyces cavourensis strain RD8 using response surface optimization. Eviron. Pollut. Prot. 2017, 2, 168–178. [Google Scholar]
- El-Naggar, N.E.A.; El-Ewasy, S.M. Bioproduction, characterization, anticancer and antioxidant activities of extracellular melanin pigment produced by newly isolated microbial cell factories Streptomyces glaucescens NEAE-H. Sci. Rep. 2017, 7, 42129. [Google Scholar] [CrossRef]
- Madhusudhan, D.; Mazhari, B.B.Z.; Dastager, S.G.; Agsar, D. Production and cytotoxicity of extracellular insoluble and droplets of soluble melanin by Streptomyces lusitanus DMZ-3. BioMed Res. Int. 2014, 2014, 306895. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, Y.; Li, Y. Metal ions driven production, characterization and bioactivity of extracellular melanin from Streptomyces sp. ZL-2. Int. J. Biol. Macromol. 2019, 123, 521–530. [Google Scholar] [CrossRef]
- Tran-Ly, A.N.; Reyes, C.; Schwarze, F.W.; Ribera, J. Microbial production of melanin and its various applications. World J. Microbiol. Biotechnol. 2020, 36, 170. [Google Scholar] [CrossRef]
- El-Batal, A.I.; El-Sayyad, G.S.; El-Ghamery, A.; Gobara, M. Response surface methodology optimization of melanin production by Streptomyces cyaneus and synthesis of copper oxide nanoparticles using gamma radiation. J. Clust. Sci. 2017, 28, 1083–1112. [Google Scholar] [CrossRef]
- Tarangini, K.; Mishra, S. Production of melanin by soil microbial isolate on fruit waste extract: Two step optimization of key parameters. Biotechnol. Rep. 2014, 4, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Surwase, S.N.; Jadhav, S.B.; Phugare, S.S.; Jadhav, J.P. Optimization of melanin production by Brevundimonas sp. SGJ using response surface methodology. Biotech 2013, 3, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Zhang, X.; Sun, S.; Zhang, L.; Shan, S.; Zhu, H. Production of natural melanin by Auricularia auricula and study on its molecular structure. Food Chem. 2016, 190, 801–807. [Google Scholar] [CrossRef]
- Medeiros, W.B.; Medina, K.J.D.; Sponchiado, S.R.P. Improved natural melanin production by Aspergillus nidulans after optimization of factors involved in the pigment biosynthesis pathway. Microb. Cell Fact. 2022, 21, 278. [Google Scholar] [CrossRef]
- Ho, S.H.; Liao, J.F.; Chen, C.Y.; Chang, J.S. Combining light strategies with recycled medium to enhance the economic feasibility of phycocyanin production with Spirulina platensis. Bioresour. Technol. 2018, 247, 669–675. [Google Scholar] [CrossRef]
- Raisa, Z.; de Jesús, Q.V.; Tamara, L.; Graciela, C.; Lorena, O.M.; Claudio, R. New protein hydrolysate from Spirulina platensis used as peptone in microbiological culture media. J. Nat. Prod. Resour. 2016, 2, 71–75. [Google Scholar]
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The potential of microalgae and their biopolymers as structuring ingredients in food: A review. Biotechnol. Adv. 2019, 37, 107419. [Google Scholar] [CrossRef] [PubMed]
- Dranseikienė, D.; Balčiūnaitė-Murzienė, G.; Karosienė, J.; Morudov, D.; Juodžiukynienė, N.; Hudz, N.; Gerbutavičienė, R.J.; Savickienė, N. Cyano-Phycocyanin: Mechanisms of action on human skin and future perspectives in medicine. Plants 2022, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Maleki, H.; Soleimanpour, H.; Norouzy, A.; Zahiri, H.S.; Vali, H.; Noghabi, K.A. Development of a novel method for the purification of C-phycocyanin pigment from a local cyanobacterial strain Limnothrix sp. NS01 and evaluation of its anticancer properties. Sci. Rep. 2019, 9, 9474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venugopal, V.C.; Thakur, A.; Chennabasappa, L.K.; Mishra, G.; Singh, K.; Rathee, P.; Ranjan, A. Phycocyanin extracted from Oscillatoria minima shows antimicrobial, algicidal, and antiradical activities: In silico and in vitro analysis. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2020, 19, 240–253. [Google Scholar] [CrossRef]
- Soni, B.; Menon, D.; Vijaykumar, V.; Ghadge, R.; Dasgupta, S. Phycocyanin extraction and production of crude bio-oil from residual biomass. Ind. Biotechnol. 2022, 18, 154–161. [Google Scholar] [CrossRef]
- Ho, S.H.; Li, R.; Zhang, C.; Ge, Y.; Cao, G.; Ma, M.; Duan, X.; Wang, S.; Ren, N.Q. N-doped graphitic biochars from C-phycocyanin extracted Spirulina residue for catalytic persulfate activation toward nonradical disinfection and organic oxidation. Water Res. 2019, 159, 77–86. [Google Scholar] [CrossRef]
- Bahari, A.N.; Saari, N.; Salim, N.; Ashari, S.E. Response factorial design analysis on papain-generated hydrolysates from Actinopyga lecanora for determination of antioxidant and antityrosinase activities. Molecules 2020, 25, 2663. [Google Scholar] [CrossRef]
- Hongfei, Z.; Fengling, B.; Fang, Z.; Walczak, P.; Xiangning, J.; Bolin, Z. Characterization of soybean protein hydrolysates able to promote the proliferation of Streptococcus thermophilus ST. J. Food Sci. 2013, 78, 57–581. [Google Scholar] [CrossRef]
- Gao, M.; Song, X.; Feng, Y.; Li, W.; Cui, Q. Isolation and characterization of Aurantiochytrium species: High docosahexaenoic acid (DHA) production by the newly isolated microalga, Aurantiochytrium sp. SD116. J. Oleo Sci. 2013, 62, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Güroy, B.; Karadal, O.; Mantoğlu, S.; Cebeci, O.I. Effects of different drying methods on C-phycocyanin content of Spirulina platensis powder. Ege J. Fish. Aquat. Sci. 2017, 34, 129–132. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; AOAC: Rockville, MD, USA, 1998. [Google Scholar]
- Pratama, A.I.; Lioe, H.N.; Yuliana, N.D.; Ogawa, M. Umami compounds present in umami fraction of acid-hydrolyzed Spirulina (Spirulina platensis). Algal Res. 2022, 66, 102764. [Google Scholar] [CrossRef]
- Kwanyuen, P.; Burton, J.W. A modified amino acid analysis using PITC derivatization for soybeans with accurate determination of cysteine and half-cystine. J. Am. Oil Chem. Soc. 2010, 87, 127–132. [Google Scholar] [CrossRef]
- Santiago-Díaz, P.; Rico, M.; Rivero, A.; Santana-Casiano, M. Bioactive metabolites of microalgae from Canary Islands for functional food and feed uses. Chem. Biodivers. 2022, 19, e202200230. [Google Scholar] [CrossRef]
- Park, K.B.; Oh, S.H. Production of yogurt with enhanced levels of gamma-aminobutyric acid and valuable nutrients using lactic acid bacteria and germinated soybean extract. Bioresour. Technol. 2007, 98, 1675–1679. [Google Scholar] [CrossRef]
- Zou, Y.; Hou, X. Optimization of culture medium for production of melanin by Auricularia auricula. Food Sci. Tech. 2017, 37, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.R.Z.; Yang, T.; Man, Z.; Xu, M.; Zhang, X. High-level production of melanin by a novel isolate of Streptomyces kathirae. FEMS Microbiol. Lett. 2014, 357, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.X.M.; Lu, M.; Shi, L.; Shi, T.; Yu, M. Characterization of the physicochemical properties, antioxidant activity, and antiproliferative activity of natural melanin from S. reiliana. Sci. Rep. 2022, 12, 2110. [Google Scholar] [CrossRef]
- Krokida, M.K.; Karathanos, V.; Maroulis, Z.; Marinos-Kouris, D. Drying kinetics of some vegetables. J. Food Eng. 2003, 59, 391–403. [Google Scholar] [CrossRef]
- Unterlander, N.; Champagne, P.; Plaxton, W.C. Lyophilization pretreatment facilitates extraction of soluble proteins and active enzymes from the oil-accumulating microalga Chlorella vulgaris. Algal Res. 2017, 25, 439–444. [Google Scholar] [CrossRef]
- Adjali, A.; Clarot, I.; Chen, Z.; Marchioni, E.; Boudier, A. Physicochemical degradation of phycocyanin and means to improve its stability: A short review. J. Pharm. Anal. 2022, 12, 406–414. [Google Scholar] [CrossRef] [PubMed]
- AlFadhly, N.K.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F.; Narayanankutty, A. Trends and technological advancements in the possible food applications of Spirulina and their health benefits: A Review. Molecules 2022, 27, 5584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Abbaspourrad, A. Improvement of the colloidal stability of phycocyanin in acidified conditions using whey protein-phycocyanin interactions. Food Hydrocoll. 2020, 105, 105747. [Google Scholar] [CrossRef]
- Yücetepe, A.; Saroğlu, Ö.; Daşkaya Dikmen, C.; Bildik, F.; Özçelik, B. Optimisation of ultrasound-assisted extraction of protein from Spirulina platensis using RSM. Czech J. Food Sci. 2018, 36, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Rafiqul, I.; Jalal, K.; Alam, M. Environmental factors for optimization of Spirulina biomass in laboratory culture. Biotechnology 2005, 4, 19–22. [Google Scholar]
- Madkour, F.F.; Kamil, A.E.W.; Nasr, H.S. Production and nutritive value of Spirulina platensis in reduced cost media. Egypt. J. Aquat. Res. 2012, 38, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Agustini, T.W.; Suzery, M.; Sutrisnanto, D.; Maruf, W.F. Comparative study of bioactive substances extracted from fresh and dried Spirulina sp. Procedia Environ. Sci. 2015, 23, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Eid, S.M.; Farag, M.A.; Bawazeer, S. Underivatized Amino Acid Chromatographic Separation: Optimized Conditions for HPLC-UV Simultaneous Quantification of Isoleucine, Leucine, Lysine, Threonine, Histidine, Valine, Methionine, Phenylalanine, Tryptophan, and Tyrosine in Dietary Supplements. ACS Omega 2022, 7, 31106–31114. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Mitsikaris, P.D.; Papadopoulos, A.N.; Samanidou, V.F. Microwave-Assisted Extraction Coupled to HPLC-UV Combined with Chemometrics for the Determination of Bioactive Compounds in Pistachio Nuts and the Guarantee of Quality and Authenticity. Molecules 2022, 27, 1435. [Google Scholar] [CrossRef]
- Holland, T.; Abdul-Munaim, A.M.; Mandrell, C.; Karunanithy, R.; Watson, D.G.; Sivakumar, P. UV-Visible Spectrophotometer for Distinguishing Oxidation Time of Engine Oil. Lubricants 2021, 9, 37. [Google Scholar] [CrossRef]
- Anema, S.G. Heat-induced changes in caseins and casein micelles, including interactions with denatured whey proteins. Int. Dairy J. 2021, 122, 105136. [Google Scholar] [CrossRef]
- Darragh, A.J.; Moughan, P.J. The effect of hydrolysis time on amino acid analysis. J. AOAC Int. 2019, 88, 888–893. [Google Scholar] [CrossRef] [Green Version]
- Montevecchi, G.; Santunione, G.; Licciardello, F.; Köker, Ö.; Masino, F.; Antonelli, A. Enrichment of wheat flour with Spirulina evaluation of thermal damage to essential amino acids during bread preparation. Food Res. Int. 2022, 157, 111357. [Google Scholar] [CrossRef] [PubMed]
- Srinuanpan, S.; Cheirsilp, B.; Prasertsan, P.; Kato, Y.; Asano, Y. Photoautotrophic cultivation of oleaginous microalgae and co-pelletization with filamentous fungi for cost-effective harvesting process and improved lipid yield. Aquac. Int. 2018, 26, 1493–1509. [Google Scholar] [CrossRef]
- Hewedy, M.; Ashour, S. Production of a melanin like pigment by Kluyveromyces marxianus and Streptomyces chibaensis. Aust. J. Basic Appl. Sci. 2009, 3, 920–927. [Google Scholar]
- Vasanthabharathi, V.; Lakshminarayanan, R.; Jayalakshmi, S. Melanin production from marine Streptomyces. Afr. J. Biotechnol. 2011, 10, 11224. [Google Scholar]
- Chandel, M.; Azmi, W. Optimization of process parameters for the production of tyrosine phenol lyase by Citrobacter freundii MTCC 2424. Bioresour. Technol. 2009, 100, 1840–1846. [Google Scholar] [CrossRef]
- Ito, S.; Kolbe, L.; Weets, G.; Wakamatsu, K. Visible light accelerates the ultraviolet A-induced degradation of eumelanin and pheomelanin. Pigment Cell Melanoma Res. 2019, 32, 441–447. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Ito, S. Advanced chemical methods in melanin determination. Pigment Cell Res. 2002, 15, 174–183. [Google Scholar] [CrossRef]
- Amal, A.M.; Abeer, K.A.; Samia, H.M.; Nadia, A.; Ahmed, K.A.; El-Hennawi, H.M. Selection of pigment (melanin) production in Streptomyces and their application in printing and dyeing of wool fabrics. Res. J. Chem. Sci. 2011, 1, 22–28. [Google Scholar]
- Harada, R.; Nomura, T.; Yamada, K.; Mochida, K.; Suzuki, K. Genetic engineering strategies for Euglena gracilis and its industrial contribution to sustainable development goals: A review. Front. Bioeng. Biotechnol. 2020, 8, 790. [Google Scholar] [CrossRef]




| Factor | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Yeast extract (g/L) | 1.40 | 3.7 | 6.00 |
| Soluble starch (g/L) | 1.00 | 3.0 | 5.00 |
| HAO-DBRH (g/L) | 0 | 18.67 | 37.34 |
| CuSO4 (g/L) | 0.0072 | 0.0136 | 0.02 |
| Composition | Samples | |
|---|---|---|
| F-DBR | HAO-DBR | |
| Moisture (%) | 5.92 | 5.83 |
| Ash (%) | 7.38 | 7.73 |
| Lipids (%) | 5.20 | 5.13 |
| Proteins (%) | 69.1 | 68.7 |
| Carbohydrates (%) | 12.4 | 12.61 |
| Total composition (%) | 100 | 100 |
| Run | Actual Variables | Actual Responses | |||
|---|---|---|---|---|---|
| Yeast Extract (g/L) | Soluble Starch (g/L) | HAO-DBRH (g/L) | CuSO4 (g/L) | Melanin Production (g/L) | |
| 1 | 1.4 | 5.0 | 0.0 | 0.02 | 0.013 |
| 2 | 1.4 | 5.0 | 0.0 | 0.0072 | 0.016 |
| 3 | 3.7 | 3.0 | 18.67 | 0.0072 | 0.123 |
| 4 | 3.7 | 1.0 | 18.67 | 0.0136 | 0.161 |
| 5 | 6.0 | 5.0 | 37.34 | 0.02 | 0.097 |
| 6 | 6.0 | 5.0 | 0.0 | 0.02 | 0.134 |
| 7 | 6.0 | 1.0 | 37.34 | 0.0072 | 0.106 |
| 8 | 3.7 | 3.0 | 0.0 | 0.0136 | 0.098 |
| 9 | 6.0 | 5.0 | 37.34 | 0.0072 | 0.074 |
| 10 | 3.7 | 5.0 | 18.67 | 0.0136 | 0.150 |
| 11 | 3.7 | 3.0 | 37.34 | 0.0136 | 0.080 |
| 12 | 6.0 | 1.0 | 37.34 | 0.02 | 0.078 |
| 13 | 3.7 | 3.0 | 18.67 | 0.0136 | 0.095 |
| 14 | 1.4 | 5.0 | 37.34 | 0.0072 | 0.053 |
| 15 | 6.0 | 1.0 | 0.0 | 0.0072 | 0.108 |
| 16 | 1.4 | 1.0 | 37.34 | 0.02 | 0.027 |
| 17 | 3.7 | 3.0 | 18.67 | 0.0136 | 0.118 |
| 18 | 6.0 | 3.0 | 18.67 | 0.0136 | 0.130 |
| 19 | 6.0 | 5.0 | 0.0 | 0.0072 | 0.116 |
| 20 | 1.4 | 5.0 | 37.34 | 0.02 | 0.076 |
| 21 | 1.4 | 1.0 | 0.0 | 0.0072 | 0.021 |
| 22 | 3.7 | 3.0 | 18.67 | 0.02 | 0.136 |
| 23 | 1.4 | 3.0 | 18.67 | 0.0136 | 0.048 |
| 24 | 1.4 | 1.0 | 0.0 | 0.02 | 0.004 |
| 25 | 3.7 | 3.0 | 18.67 | 0.0136 | 0.112 |
| 26 | 6.0 | 1.0 | 0.0 | 0.02 | 0.134 |
| 27 | 1.4 | 1.0 | 37.34 | 0.0072 | 0.055 |
| Source | SS | Df | MS | F-Value | p > F |
|---|---|---|---|---|---|
| Model | 0.05 | 14 | 3.545 × 10−3 | 15.38 | <0.0001 |
| A: Yeast extract | 0.024 | 1 | 0.024 | 106.27 | <0.0001 |
| B: Soluble starch | 6.806 × 10−5 | 1 | 6.806 × 10−5 | 0.30 | 0.5968 |
| C: HAO-DBRH | 2.222 × 10−7 | 1 | 2.222 × 10−7 | 9.642 × 10−4 | 0.9757 |
| D: CuSO4 | 4.050 × 10−5 | 1 | 4.050 × 10−5 | 0.18 | 0.6825 |
| A2 | 4.038 × 10−3 | 1 | 4.038 × 10−3 | 17.52 | 0.0013 |
| B2 | 1.857 × 10−3 | 1 | 1.857 × 10−3 | 8.06 | 0.0149 |
| C2 | 4.038 × 10−3 | 1 | 4.038 × 10−3 | 17.52 | 0.0013 |
| D2 | 1.948 × 10−6 | 1 | 1.948 × 10−6 | 8.452 × 10−3 | 0.9283 |
| AB | 1.960 × 10−4 | 1 | 1.960 × 10−4 | 0.85 | 0.3746 |
| AC | 5.402 × 10−3 | 1 | 5.402 × 10−3 | 23.44 | 0.004 |
| AD | 2.560 × 10−4 | 1 | 2.560 × 10−4 | 1.11 | 0.3127 |
| BC | 3.025 × 10−5 | 1 | 3.025 × 10−5 | 0.13 | 0.7234 |
| BD | 7.290 × 10−4 | 1 | 7.290 × 10−4 | 3.16 | 0.1007 |
| CD | 7.225 × 10−5 | 1 | 7.225 × 10−5 | 0.31 | 0.5859 |
| Residual | 2.766 × 10−3 | 12 | 2.766 × 10−3 | ||
| Lack of Fit | 2.481 × 10−3 | 10 | 2.481 × 10−3 | 1.74 | 0.4190 |
| Pure Error | 2.847 × 10−4 | 2 | 1.423 × 10−3 | ||
| Cor Total | 0.052 | 26 | |||
| R2 | 0.9472 | ||||
| C.V. | 17.35 |
| Conditions | Yeast Extract (g/L) | Soluble Starch (g/L) | CuSO4 | NaCl (g/L) | CaCl2 (g/L) | HAO-DBRH | Predicted Value | Actual Value |
|---|---|---|---|---|---|---|---|---|
| (g/L) | (g/L) | (g/L) | (g/L) | |||||
| OM1 | 4.65 | 4.98 | 0.0179 | 5 | 0.1 | 17.89 | 0.164 | 0.172 |
| OM2 | 4.14 | 4.98 | 0.0198 | 5 | 0.1 | 17.30 | 0.165 | 0.125 |
| OM3 | 5.16 | 4.92 | 0.0191 | 5 | 0.1 | 17.11 | 0.164 | 0.244 |
| Un-OM | 37 | 3.3 | 0.0136 | 5 | 0.1 | - | - | 0.061 |
| OM1 | OM2 | OM3 | Un-OM | |
|---|---|---|---|---|
| Yeast extract | 0.74 | 0.66 | 0.82 | 5.86 |
| Soluble starch | 0.25 | 0.25 | 0.25 | 0.17 |
| CuSO4 | 0.0001 | 0.00012 | 0.00011 | 0.00008 |
| NaCl | 0.195 | 0.195 | 0.195 | 0.195 |
| CaCl2 | 0.004 | 0.004 | 0.004 | 0.004 |
| HAO-DBRH | 0 | 0 | 0 | 0 |
| HCl | 8.82 | 8.82 | 8.82 | 0 |
| Medium cost (USD/L) | 1.19 | 1.11 | 1.25 | 6.22 |
| Experimentally melanin production (g/L) | 1.72 | 1.25 | 2.44 | 0.61 |
| Medium cost (USD/g melanin) | 0.69 | 0.89 | 0.52 | 10.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraseasintra, O.; Sensupa, S.; Mahanil, K.; Yoosathaporn, S.; Pekkoh, J.; Srinuanpan, S.; Pathom-aree, W.; Pumas, C. Optimization of Melanin Production by Streptomyces antibioticus NRRL B-1701 Using Arthrospira (Spirulina) platensis Residues Hydrolysates as Low-Cost L-tyrosine Supplement. BioTech 2023, 12, 24. https://doi.org/10.3390/biotech12010024
Kraseasintra O, Sensupa S, Mahanil K, Yoosathaporn S, Pekkoh J, Srinuanpan S, Pathom-aree W, Pumas C. Optimization of Melanin Production by Streptomyces antibioticus NRRL B-1701 Using Arthrospira (Spirulina) platensis Residues Hydrolysates as Low-Cost L-tyrosine Supplement. BioTech. 2023; 12(1):24. https://doi.org/10.3390/biotech12010024
Chicago/Turabian StyleKraseasintra, Oranit, Sritip Sensupa, Kanjana Mahanil, Sada Yoosathaporn, Jeeraporn Pekkoh, Sirasit Srinuanpan, Wasu Pathom-aree, and Chayakorn Pumas. 2023. "Optimization of Melanin Production by Streptomyces antibioticus NRRL B-1701 Using Arthrospira (Spirulina) platensis Residues Hydrolysates as Low-Cost L-tyrosine Supplement" BioTech 12, no. 1: 24. https://doi.org/10.3390/biotech12010024
APA StyleKraseasintra, O., Sensupa, S., Mahanil, K., Yoosathaporn, S., Pekkoh, J., Srinuanpan, S., Pathom-aree, W., & Pumas, C. (2023). Optimization of Melanin Production by Streptomyces antibioticus NRRL B-1701 Using Arthrospira (Spirulina) platensis Residues Hydrolysates as Low-Cost L-tyrosine Supplement. BioTech, 12(1), 24. https://doi.org/10.3390/biotech12010024