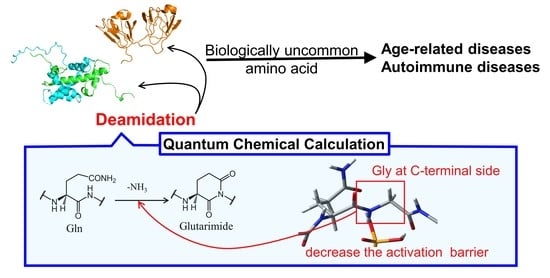
Nonenzymatic Deamidation Mechanism on a Glutamine Residue with a C-Terminal Adjacent Glycine Residue: A Computational Mechanistic Study
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussion
3.1. Comparison of the Optimized Geometries in the Cyclization Step
3.2. Comparison of the Optimized Geometries in the Deammoniation Step
3.3. Energy Profiles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geiger, T.; Clarke, S. Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J. Biol. Chem. 1987, 262, 785–794. [Google Scholar] [CrossRef]
- Lindner, H.; Helliger, H. Age-dependent deamidation of asparagine residues in proteins. Exp. Gerontol. 2001, 36, 1551–1563. [Google Scholar] [CrossRef]
- Robinson, N.E.; Robinson, A.B. Molecular clocks. Proc. Natl. Acad. Sci. USA 2001, 98, 944–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, N.E. Protein deamidation. Proc. Natl. Acad. Sci. USA 2002, 99, 5283–5288. [Google Scholar] [CrossRef] [Green Version]
- Lampi, K.J.; Wilmarth, P.A.; Murray, M.R.; David, L.L. Lens β-crystallins: The role of deamidation and related modifications in aging and cataract. Prog. Biophys. Mol. Biol. 2014, 115, 21–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, W.G.; Papaconstantinou, J. Aging of α-crystallins during development of the lens. Proc. Natl. Acad. Sci. USA 1969, 64, 404–410. [Google Scholar] [CrossRef] [Green Version]
- Takemoto, L.; Boyle, D. Deamidation of specific glutamine residues from α-A crystallin during aging of the human lens. Biochemistry 1998, 37, 13681–13685. [Google Scholar] [CrossRef] [PubMed]
- Lapko, V.N.; Purkiss, A.G.; Smith, D.L.; Smith, J.B. Deamidation in human gamma S-crystallin from cataractous lenses is influenced by surface exposure. Biochemistry 2002, 41, 8638–8648. [Google Scholar] [CrossRef]
- Patel, K.; Borchardt, R.T. Chemical pathways of peptide degradation. II. Kinetics of deamidation of an asparaginyl residue in a model hexapeptide. Pharm. Res. 1990, 7, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, C.; O’Connor, P.B. Glutamine deamidation: Differentiation of glutamic acid and gamma-glutamic acid in peptides by electron capture dissociation. Anal. Chem. 2010, 82, 3606–3615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capasso, S.; Mazzarella, L.; Sica, F.; Zagari, A. First evidence of spontaneous deamidation of glutamine residue via cyclic imide to α- and γ-glutamic residue under physiological conditions. J. Chem. Soc. Chem. Commun. 1991, 23, 1667–1668. [Google Scholar] [CrossRef]
- Moss, C.X.; Matthews, S.P.; Lamont, D.J.; Watts, C. Asparagine deamidation perturbs antigen presentation on class II major histocompatibility complex molecules. J. Biol. Chem. 2005, 280, 18498–18503. [Google Scholar] [CrossRef] [Green Version]
- Doyle, H.A.; Gee, R.J.; Mamula, M.J. Altered immunogenicity of isoaspartate containing proteins. Autoimmunity 2007, 40, 131–137. [Google Scholar] [CrossRef]
- Doyle, H.A.; Aswad, D.W.; Mamula, M.J. Autoimmunity to isomerized histone H2B in systemic lupus erythematosus. Autoimmunity 2013, 46, 6–13. [Google Scholar] [CrossRef]
- Qin, Z.; Zhu, J.X.; Aswad, D.W. The d-isoAsp-25 variant of histone H2B is highly enriched in active chromatin: Potential role in the regulation of gene expression? Amino Acids 2016, 48, 599–603. [Google Scholar] [CrossRef] [Green Version]
- Diós, Á.; Elek, R.; Szabó, I.; Horváth, S.; Gyimesi, J.; Király, R.; Werkstetter, K.; Koletzko, S.; Fésüs, L.; Korponay-Szabó, I.R. Gamma-gliadin specific celiac disease antibodies recognize p31-43 and p57-68 alpha gliadin peptides in deamidation related manner as a result of cross-reaction. Amino Acids 2021, 53, 1051–1063. [Google Scholar] [CrossRef]
- Lerner, A.; Benzvi, C. Microbial transglutaminase is a very frequently used food additive and is a potential inducer of autoimmune/neurodegenerative diseases. Toxics 2021, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- van Lummel, M.; Duinkerken, G.; van Veelen, P.A.; de Ru, A.; Cordfunke, R.; Zaldumbide, A.; Gomez-Touriño, I.; Arif, S.; Peakman, M.; Drijfhout, J.W.; et al. Posttranslational modification of HLA-DQ binding islet autoantigens in type 1 diabetes. Diabetes 2014, 63, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Boswell, C.A.; Tesar, D.B.; Mukhyala, K.; Theil, F.P.; Fielder, P.J.; Khawli, L.A. Effects of charge on antibody tissue distribution and pharmacokinetics. Bioconjug. Chem. 2010, 21, 2153–2163. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.D.; van Enk, J.Z.; Flynn, G.C. Human antibody Fc deamidation in vivo. Biologicals 2009, 37, 313–322. [Google Scholar] [CrossRef]
- Harris, R.J.; Kabakoff, B.; Macchi, F.D.; Shen, F.J.; Kwong, M.; Andya, J.D.; Shire, S.J.; Bjork, N.; Totpal, K.; Chen, A.B. Identification of multiple sources of charge heterogeneity in a recombinant antibody. J. Chromatogr. B Biomed. Sci. Appl. 2001, 752, 233–245. [Google Scholar] [CrossRef]
- Tran, J.C.; Tran, D.; Hilderbrand, A.; Andersen, N.; Huang, T.; Reif, K.; Hotzel, I.; Stefanich, E.G.; Liu, Y.; Wang, J. Automated affinity capture and on-tip digestion to accurately quantitate in vivo deamidation of therapeutic antibodies. Anal. Chem. 2016, 88, 11521–11526. [Google Scholar] [CrossRef]
- Du, Y.; Walsh, A.; Ehrick, R.; Xu, W.; May, K.; Liu, H. Chromatographic analysis of the acidic and basic species of recombinant monoclonal antibodies. MAbs 2012, 4, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.E.; Robinson, A.B. Molecular Clocks: Deamidation of Asparaginyl and Glutaminyl Residues in Peptides and Proteins; Althouse Press: Cave Junction, OR, USA, 2004. [Google Scholar]
- Robinson, N.E.; Robinson, Z.W.; Robinson, B.R.; Robinson, A.L.; Robinson, J.A.; Robinson, M.L.; Robinson, A.B. Structure-dependent non-enzymatic deamidation of glutaminyl and asparaginyl pentapeptides. J. Pept. Res. 2004, 63, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Scotchler, J.W.; Robinson, A.B. Deamidation of glutaminyl residues: Dependence on pH, temperature, and ionic strength. Anal. Biochem. 1974, 59, 319–322. [Google Scholar] [CrossRef]
- Pace, A.L.; Wong, R.L.; Zhang, Y.T.; Kao, Y.H.; Wang, Y.J. Asparagine deamidation dependence on buffer type, pH, and temperature. J. Pharm. Sci. 2013, 102, 1712–1723. [Google Scholar] [CrossRef]
- Kato, K.; Nakayoshi, T.; Kurimoto, E.; Oda, A. Computational studies on the non-enzymatic deamidation mechanisms of Glutamine residues. ACS Omega 2019, 4, 3508–3513. [Google Scholar] [CrossRef]
- Manabe, N.; Kirikoshi, R.; Takahashi, O. Glycolic acid-catalyzed deamidation of asparagine residues in degrading PLGA matrices: A computational study. Int. J. Mol. Sci. 2015, 16, 7261–7272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, O.; Manabe, N.; Kirikoshi, R. A computational study of the mechanism of succinimide formation in the Asn-His sequence: Intramolecular catalysis by the His side chain. Molecules 2016, 21, 327. [Google Scholar] [CrossRef] [Green Version]
- Kirikoshi, R.; Manabe, N.; Takahashi, O. Succinimide formation from an NGR-containing cyclic peptide: Computational evidence for catalytic roles of phosphate buffer and the arginine side chain. Int. J. Mol. Sci. 2017, 18, 429. [Google Scholar] [CrossRef] [Green Version]
- Nakayoshi, T.; Kato, K.; Kurimoto, E.; Oda, A. Possible mechanisms of non-enzymatic formation of dehydroalanine residue catalyzed by dihydrogen phosphate ion. J. Phys. Chem. B 2019, 123, 3147–3155. [Google Scholar] [CrossRef] [PubMed]
- Halim, M.A.; Almatarneh, M.H.; Poirier, R.A. Mechanistic study of the deamidation reaction of glutamine: A computational approach. J. Phys. Chem. B 2014, 118, 2316–2330. [Google Scholar] [CrossRef] [PubMed]
- Nakayoshi, T.; Kato, K.; Fukuyoshi, S.; Takahashi, O.; Kurimoto, E.; Oda, A. Molecular mechanisms of succinimide formation from aspartic acid residues catalyzed by two water molecules in the aqueous phase. Int. J. Mol. Sci. 2021, 22, 509. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Nakayoshi, T.; Ishikawa, Y.; Kurimoto, E.; Oda, A. Computational analysis of the mechanism of non-enzymatic peptide bond cleavage at the C-terminal side of an asparagine residue. ACS Omega 2021, 6, 30078–30084. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Hooi, M.Y.; Raftery, M.J.; Truscott, R.J. Age-dependent deamidation of glutamine residues in human γS crystallin: Deamidation and unstructured regions. Protein Sci. 2012, 21, 1074–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, K.; Nakayoshi, T.; Kurimoto, E.; Oda, A. Mechanisms of deamidation of asparagine residues and effects of main-chain conformation on activation energy. Int. J. Mol. Sci. 2020, 21, 7035. [Google Scholar] [CrossRef]
- Connolly, B.D.; Tran, B.; Moore, J.M.R.; Sharma, V.K.; Kosky, A. Specific catalysis of asparaginyl deamidation by carboxylic acids: Kinetic, thermodynamic, and quantitative structure-property relationship analyses. Mol. Pharm. 2014, 11, 1345–1358. [Google Scholar] [CrossRef] [PubMed]













| φ | ψ | χ1 | χ2 | |
|---|---|---|---|---|
| RC-A | −150 | 145 | −173 | −79.6 |
| TS1-A | −157 | −178 | 179 | −66.4 |
| INT1-A | −155 | −157 | 176 | −63.8 |
| RC-B | −150 | 136 | −167 | −70.2 |
| TS1-B | −157 | −177 | −178 | −68.2 |
| INT1-B | −155 | −154 | 172 | −61.0 |
| RC-C | −147 | 141 | −177 | −75.4 |
| TS1-C | −155 | 169 | 172 | −57.1 |
| INT1-C | −106 | −148 | 177 | −64.0 |
| Chain A | Chain B | |||||||
|---|---|---|---|---|---|---|---|---|
| φ | ψ | χ1 | χ2 | φ | ψ | χ1 | χ2 | |
| Gln16 | −118 | 158 | −58.4 | −70.2 | −79.3 | 168 | −57.9 | −48.2 |
| Gln63 | −52.5 | 128 | −174 | 166 | −47.9 | 135 | 165 | 173 |
| Gln92 | −101 | 135 | −103 | 51.4 | −87.9 | 138 | −130 | 60.2 |
| Gln120 | −93.7 | −31 | −174 | 180 | −87.4 | −27.2 | −171 | 177 |
| φ | ψ | χ1 | χ2 | |
|---|---|---|---|---|
| INT2 | −107 | −152 | 173 | −60.4 |
| TS2 | −107 | −153 | 174 | −58.8 |
| PC | −99.6 | −163 | −179 | −48.8 |
| HF | B3LYP | CAM-B3LYP | ωB97XD | MP2 | |
|---|---|---|---|---|---|
| RC | 0 | 0.0 | 0 | 0 | 0.0 |
| TS1 | 161 | 115 | 115 | 113 | 96.9 |
| INT1 | 90.1 | 91.3 | 78.9 | 74.1 | 53.9 |
| INT2 | 81.7 | 71.1 | 61.9 | 63.9 | 49.2 |
| TS2 | 139 | 89.2 | 85.1 | 89.3 | 74.2 |
| PC | 18.7 | 31.8 | 35.9 | 49.1 | 37.9 |
| HF | B3LYP | CAM-B3LYP | ωB97XD | MP2 | |
|---|---|---|---|---|---|
| RC | 0 | 0 | 0 | 0 | 0 |
| TS1 | 156 | 111 | 111 | 106 | 85.4 |
| INT1 | 89.6 | 89.0 | 78.6 | 77.9 | 55.8 |
| INT2 | 91.2 | 76.5 | 65.8 | 63.0 | 46.5 |
| TS2 | 148 | 94.3 | 89.0 | 89.1 | 71.7 |
| PC | 19.8 | 38.5 | 41.0 | 46.5 | 29.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asai, H.; Kato, K.; Nakayoshi, T.; Ishikawa, Y.; Kurimoto, E.; Oda, A.; Fukuishi, N. Nonenzymatic Deamidation Mechanism on a Glutamine Residue with a C-Terminal Adjacent Glycine Residue: A Computational Mechanistic Study. AppliedChem 2021, 1, 142-155. https://doi.org/10.3390/appliedchem1020011
Asai H, Kato K, Nakayoshi T, Ishikawa Y, Kurimoto E, Oda A, Fukuishi N. Nonenzymatic Deamidation Mechanism on a Glutamine Residue with a C-Terminal Adjacent Glycine Residue: A Computational Mechanistic Study. AppliedChem. 2021; 1(2):142-155. https://doi.org/10.3390/appliedchem1020011
Chicago/Turabian StyleAsai, Haruka, Koichi Kato, Tomoki Nakayoshi, Yoshinobu Ishikawa, Eiji Kurimoto, Akifumi Oda, and Nobuyuki Fukuishi. 2021. "Nonenzymatic Deamidation Mechanism on a Glutamine Residue with a C-Terminal Adjacent Glycine Residue: A Computational Mechanistic Study" AppliedChem 1, no. 2: 142-155. https://doi.org/10.3390/appliedchem1020011
APA StyleAsai, H., Kato, K., Nakayoshi, T., Ishikawa, Y., Kurimoto, E., Oda, A., & Fukuishi, N. (2021). Nonenzymatic Deamidation Mechanism on a Glutamine Residue with a C-Terminal Adjacent Glycine Residue: A Computational Mechanistic Study. AppliedChem, 1(2), 142-155. https://doi.org/10.3390/appliedchem1020011