Effect of Metal Dopant on the Performance of Ni@CeMeO2 Embedded Catalysts (Me = Gd, Sm and Zr) for Dry Reforming of Methane
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. DRM Reaction
2.3. Characterization of Spent Catalysts
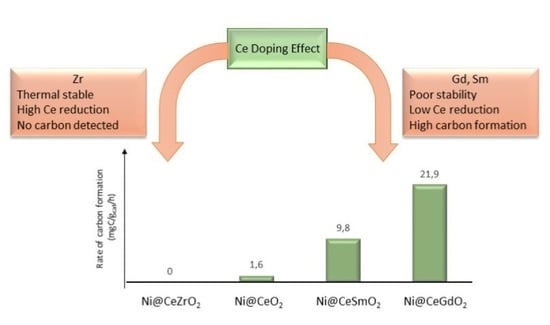
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Characterization
4.2.1. X-ray Fluorescence
4.2.2. N2 Adsorption
4.2.3. In Situ X-ray Diffraction
4.2.4. Raman Spectroscopy
4.2.5. Temperature-Programmed Reduction
4.2.6. 18O2/16O2 Isotopic Exchange
4.3. Catalytic Test
4.4. Carbon Analysis
4.4.1. Scanning Electron Microscopy
4.4.2. Thermogravimetric Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- González, M.O.A.; Gonçalves, J.S.; Vasconcelos, R.M. Sustainable Development: Case Study in the Implementation of Renewable Energy in Brazil. J. Clean. Prod. 2017, 142, 461–475. [Google Scholar] [CrossRef]
- Ullah Khan, I.; Hafiz Dzarfan Othman, M.; Hashim, H.; Matsuura, T.; Ismail, A.F.; Rezaei-DashtArzhandi, M.; Wan Azelee, I. Biogas as a Renewable Energy Fuel—A Review of Biogas Upgrading, Utilisation and Storage. Energy Convers. Manag. 2017, 150, 277–294. [Google Scholar] [CrossRef]
- Yang, L.; Ge, X.; Wan, C.; Yu, F.; Li, Y. Progress and Perspectives in Converting Biogas to Transportation Fuels. Renew. Sustain. Energy Rev. 2014, 40, 1133–1152. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A Review of Recent Efforts to Promote Dry Reforming of Methane (DRM) to Syngas Production via Bimetallic Catalyst Formulations. Appl. Catal. B Environ. 2021, 296, 120210. [Google Scholar] [CrossRef]
- Shi, C.; Wang, S.; Ge, X.; Deng, S.; Chen, B.; Shen, J. A Review of Different Catalytic Systems for Dry Reforming of Methane: Conventional Catalysis-Alone and Plasma-Catalytic System. J. CO2 Util. 2021, 46, 101462. [Google Scholar] [CrossRef]
- Marinho, A.L.A.; Rabelo-Neto, R.C.; Epron, F.; Bion, N.; Toniolo, F.S.; Noronha, F.B. Embedded Ni Nanoparticles in CeZrO2 as Stable Catalyst for Dry Reforming of Methane. Appl. Catal. B Environ. 2020, 268, 118387. [Google Scholar] [CrossRef]
- Marinho, A.L.A.; Rabelo-Neto, R.C.; Epron, F.; Bion, N.; Noronha, F.B.; Toniolo, F.S. Pt Nanoparticles Embedded in CeO2 and CeZrO2 Catalysts for Biogas Upgrading: Investigation on Carbon Removal Mechanism by Oxygen Isotopic Exchange and DRIFTS. J. CO2 Util. 2021, 49, 101572. [Google Scholar] [CrossRef]
- Marinho, A.L.A.; Toniolo, F.S.; Noronha, F.B.; Epron, F.; Duprez, D.; Bion, N. Highly Active and Stable Ni Dispersed on Mesoporous CeO2-Al2O3 Catalysts for Production of Syngas by Dry Reforming of Methane. Appl. Catal. B Environ. 2021, 281, 119459. [Google Scholar] [CrossRef]
- Bian, Z.; Kawi, S. Sandwich-Like Silica@Ni@Silica Multicore-Shell Catalyst for the Low-Temperature Dry Reforming of Methane: Confinement Effect Against Carbon Formation. ChemCatChem 2018, 10, 320–328. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Shi, W. Syngas Production from CO2 Reforming with Methane over Core-Shell Ni@SiO2 Catalysts. J. CO2 Util. 2016, 16, 318–327. [Google Scholar] [CrossRef]
- Pu, J.; Luo, Y.; Wang, N.; Bao, H.; Wang, X.; Qian, E.W. Ceria-Promoted Ni@Al 2 O 3 Core-Shell Catalyst for Steam Reforming of Acetic Acid with Enhanced Activity and Coke Resistance. Int. J. Hydrogen Energy 2018, 43, 3142–3153. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F. Coke-Resistant Ni@SiO2 Catalyst for Dry Reforming of Methane. Appl. Catal. B Environ. 2015, 176–177, 513–521. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Li, J.; Ye, Z.; Hu, X.; Dong, D. Electrospun Nanofibrous Ni/LaAlO3 Catalysts for Syngas Production by High Temperature Methane Partial Oxidation. Int. J. Hydrogen Energy 2022, 47, 3867–3875. [Google Scholar] [CrossRef]
- Li, Z.; Mo, L.; Kathiraser, Y.; Kawi, S. Yolk–Satellite–Shell Structured Ni–Yolk@Ni@SiO2 Nanocomposite: Superb Catalyst toward Methane CO2 Reforming Reaction. ACS Catal. 2014, 4, 1526–1536. [Google Scholar] [CrossRef]
- Han, J.W.; Kim, C.; Park, J.S.; Lee, H. Highly Coke-Resistant Ni Nanoparticle Catalysts with Minimal Sintering in Dry Reforming of Methane. ChemSusChem 2014, 7, 451–456. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Sebastian, V.; Monzon, A.; Baker, M.A.; Hinder, S.J.; Polychronopoulou, K.; Yentekakis, I.V.; Goula, M.A. An in Depth Investigation of Deactivation through Carbon Formation during the Biogas Dry Reforming Reaction for Ni Supported on Modified with CeO2 and La2O3 Zirconia Catalysts. Int. J. Hydrogen Energy 2018, 43, 18955–18976. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An Overview on Dry Reforming of Methane: Strategies to Reduce Carbonaceous Deactivation of Catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Padi, S.P.; Shelly, L.; Komarala, E.P.; Schweke, D.; Hayun, S.; Rosen, B.A. Coke-Free Methane Dry Reforming over Nano-Sized NiO-CeO2 Solid Solution after Exsolution. Catal. Commun. 2020, 138, 105951. [Google Scholar] [CrossRef]
- Liu, Z.; Grinter, D.C.; Lustemberg, P.G.; Nguyen-Phan, T.D.; Zhou, Y.; Luo, S.; Waluyo, I.; Crumlin, E.J.; Stacchiola, D.J.; Zhou, J.; et al. Dry Reforming of Methane on a Highly-Active Ni-CeO2 Catalyst: Effects of Metal-Support Interactions on C-H Bond Breaking. Angew. Chem. Int. Ed. Engl. 2016, 55, 7455–7459. [Google Scholar] [CrossRef] [Green Version]
- Luisetto, I.; Tuti, S.; Romano, C.; Boaro, M.; Di Bartolomeo, E. Dry Reforming of Methane over Ni Supported on Doped CeO2: New Insight on the Role of Dopants for CO2 Activation. J. CO2 Util. 2019, 30, 63–78. [Google Scholar] [CrossRef]
- Löfberg, A.; Guerrero-Caballero, J.; Kane, T.; Rubbens, A.; Jalowiecki-Duhamel, L. Ni/CeO2 Based Catalysts as Oxygen Vectors for the Chemical Looping Dry Reforming of Methane for Syngas Production. Appl. Catal. B Environ. 2017, 212, 159–174. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Stagg-Williams, S.M.; Noronha, F.B.; Fendley, G.; Resasco, D.E. CO2 Reforming of CH4 over Pt/ZrO2 Catalysts Promoted with La and Ce Oxides. J. Catal. 2000, 194, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Noronha, F.B.; Fendley, E.C.; Soares, R.R.; Alvarez, W.E.; Resasco, D.E. Correlation between Catalytic Activity and Support Reducibility in the CO2 Reforming of Methane over Pt/CexZr1−xO2 Catalysts. Chem. Eng. J. 2001, 82, 21–31. [Google Scholar] [CrossRef]
- Yao, H. Ceria in Automotive Exhaust Catalysts I. Oxygen Storage. J. Catal. 1984, 86, 254–265. [Google Scholar] [CrossRef]
- Pérez-Coll, D.; Marrero-López, D.; Ruiz-Morales, J.C.; Núñez, P.; Abrantes, J.C.C.; Frade, J.R. Reducibility of Ce1−xGdxO2−δ in Prospective Working Conditions. J. Power Sources 2007, 173, 291–297. [Google Scholar] [CrossRef]
- Aribi, K.; Soltani, Z.; Ghelamallah, M.; Granger, P. Structure, Morphology and Reducibility of Ceria-Doped Zirconia. J. Mol. Struct. 2018, 1156, 369–376. [Google Scholar] [CrossRef]
- Mogensen, M.; Sammes, N.M.; Tompsett, G.A. Physical, Chemical and Electrochemical Properties of Pure and Doped Ceria. Solid State Ion. 2000, 129, 63–94. [Google Scholar] [CrossRef]
- Fu, Y.-P.; Chen, S.-H.; Huang, J.-J. Preparation and Characterization of Ce0.8M0.2O2−δ (M=Y, Gd, Sm, Nd, La) Solid Electrolyte Materials for Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2010, 35, 745–752. [Google Scholar] [CrossRef]
- Nakajima, A.; Yoshihara, A.; Ishigame, M. Defect-Induced Raman Spectra in Doped Ce02. Phys. Rev. B 1994, 50, 13297. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.A.A.; Bion, N.; Epron, F.; Baraka, S.; Fonseca, F.C.; Rabelo-Neto, R.C.; Mattos, L.V.; Noronha, F.B. Effect of the Type of Ceria Dopant on the Performance of Ni/CeO 2 SOFC Anode for Ethanol Internal Reforming. Appl. Catal. B Environ. 2017, 206, 626–641. [Google Scholar] [CrossRef]
- Faria, E.C.; Neto, R.C.R.; Colman, R.C.; Noronha, F.B. Hydrogen Production through CO2 Reforming of Methane over Ni/CeZrO2/Al2O3 Catalysts. Catal. Today 2014, 228, 138–144. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 Catalysts for the Dry Reforming of Methane. Appl. Catal. A Gen. 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.; Chen, X.; Rui, N.; Betancourt, L.E.; Lin, L.; Xu, W.; Sun, C.; Abeykoon, A.M.M.; Rodriguez, J.A.; et al. Effects of Zr Doping into Ceria for the Dry Reforming of Methane over Ni/CeZrO2 Catalysts: In Situ Studies with XRD, XAFS, and AP-XPS. ACS Catal. 2020, 10, 3274–3284. [Google Scholar] [CrossRef]
- Hori, C.E.; Permana, H.; Ng, K.Y.S.; Brenner, A.; More, K.; Rahmoeller, K.M.; Belton, D. Thermal Stability of Oxygen Storage Properties in a Mixed CeO2-ZrO2 System. Appl. Catal. B Environ. 1998, 16, 105–117. [Google Scholar] [CrossRef]
- Monte, R.D.; Kašpar, J. On the Role of Oxygen Storage in Three-Way Catalysis. Top. Catal. 2004, 28, 47–57. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Hanson, J.C.; Kim, J.-Y.; Liu, G.; Iglesias-Juez, A.; Fernández-García, M. Properties of CeO2 and Ce1-XZrxO2 Nanoparticles: X-Ray Absorption Near-Edge Spectroscopy, Density Functional, and Time-Resolved X-Ray Diffraction Studies. J. Phys. Chem. B 2003, 107, 3535–3543. [Google Scholar] [CrossRef]
- Deguchi, H.; Yoshida, H.; Inagaki, T.; Horiuchi, M. EXAFS Study of Doped Ceria Using Multiple Data Set Fit. Solid State Ion. 2005, 176, 1817–1825. [Google Scholar] [CrossRef]
- Durgasri, D.N.; Vinodkumar, T.; Reddy, B.M. Facile Synthesis of Catalytically Active CeO2–Gd2O3solid Solutions for Soot Oxidation. J. Chem. Sci. 2014, 126, 429–435. [Google Scholar] [CrossRef]
- Karaca, T.; Altınçekiç, T.G.; Faruk Öksüzömer, M. Synthesis of Nanocrystalline Samarium-Doped CeO2 (SDC) Powders as a Solid Electrolyte by Using a Simple Solvothermal Route. Ceram. Int. 2010, 36, 1101–1107. [Google Scholar] [CrossRef]
- Da Fonseca, R.O.; Garrido, G.S.; Rabelo-Neto, R.C.; Silveira, E.B.; Simões, R.C.C.; Mattos, L.V.; Noronha, F.B. Study of the Effect of Gd-Doping Ceria on the Performance of Pt/GdCeO2/Al2O3 Catalysts for the Dry Reforming of Methane. Catal. Today 2020, 355, 737–745. [Google Scholar] [CrossRef]
- Bonk, A.; Remhof, A.; Maier, A.C.; Trottmann, M.; Schlupp, M.V.F.; Battaglia, C.; Vogt, U.F. Low-Temperature Reducibility of MxCe1–XO2 (M = Zr, Hf) under Hydrogen Atmosphere. J. Phys. Chem. C 2015, 120, 118–125. [Google Scholar] [CrossRef]
- Mattos, L.V.; De Oliveira, E.R.; Resende, P.D.; Noronha, F.B.; Passos, F.B. Partial Oxidation of Methane on Pt/Ce–ZrO2 Catalysts. Catal. Today 2002, 77, 245–256. [Google Scholar] [CrossRef]
- Teles, C.A.; De Souza, P.M.; Braga, A.H.; Rabelo-Neto, R.C.; Teran, A.; Jacobs, G.; Resasco, D.E.; Noronha, F.B. The Role of Defect Sites and Oxophilicity of the Support on the Phenol Hydrodeoxygenation Reaction. Appl. Catal. B Environ. 2019, 249, 292–305. [Google Scholar] [CrossRef]
- Taniguchi, T.; Watanabe, T.; Sugiyama, N.; Subramani, A.K.; Wagata, H.; Matsushita, N.; Yoshimura, M. Identifying Defects in Ceria-Based Nanocrystals by UV Resonance Raman Spectroscopy. J. Phys. Chem. C 2009, 113, 19789–19793. [Google Scholar] [CrossRef]
- Siakavelas, G.I.; Charisiou, N.D.; AlKhoori, S.; AlKhoori, A.A.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Yentekakis, I.V.; Polychronopoulou, K.; Goula, M.A. Highly Selective and Stable Nickel Catalysts Supported on Ceria Promoted with Sm2O3, Pr2O3 and MgO for the CO2 Methanation Reaction. Appl. Catal. B: Environ. 2021, 282, 119562. [Google Scholar] [CrossRef]
- Loridant, S. Raman Spectroscopy as a Powerful Tool to Characterize Ceria-Based Catalysts. Catal. Today 2021, 373, 98–111. [Google Scholar] [CrossRef]
- Da Fonseca, R.O.; Ponseggi, A.R.; Rabelo-Neto, R.C.; Simões, R.C.C.; Mattos, L.V.; Noronha, F.B. Controlling Carbon Formation over Ni/CeO2 Catalyst for Dry Reforming of CH4 by Tuning Ni Crystallite Size and Oxygen Vacancies of the Support. J. CO2 Util. 2022, 57, 101880. [Google Scholar] [CrossRef]
- Hennings, U.; Reimert, R. Investigation of the Structure and the Redox Behavior of Gadolinium Doped Ceria to Select a Suitable Composition for Use as Catalyst Support in the Steam Reforming of Natural Gas. Appl. Catal. A Gen. 2007, 325, 41–49. [Google Scholar] [CrossRef]
- Bedrane, S.; Descorme, C.; Duprez, D. Investigation of the Oxygen Storage Process on Ceria- and Ceria–Zirconia-Supported Catalysts. Catal. Today 2002, 75, 401–405. [Google Scholar] [CrossRef]
- Kuhn, M.; Bishop, S.R.; Rupp, J.L.M.; Tuller, H.L. Structural Characterization and Oxygen Nonstoichiometry of Ceria-Zirconia (Ce1−xZrxO2−δ) Solid Solutions. Acta Mater. 2013, 61, 4277–4288. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Hatzisymeon, M.; Betsi-Argyropoulou, I.; Botzolaki, G.; Kousi, K.; Kondarides, D.I.; Taylor, M.J.; Parlett, C.M.A.; Osatiashtiani, A.; et al. Effect of Support Oxygen Storage Capacity on the Catalytic Performance of Rh Nanoparticles for CO2 Reforming of Methane. Appl. Catal. B Environ. 2019, 243, 490–501. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wu, Q.; Zhang, J.; Zhang, J. Effect of Preparation Methods on Structure and Performance of Ni/Ce0.75Zr0.25O2 Catalysts for CH4–CO2 Reforming. Fuel 2008, 87, 2901–2907. [Google Scholar] [CrossRef]
- Ramírez-Cabrera, E.; Atkinson, A.; Chadwick, D. Reactivity of Ceria, Gd- and Nb-Doped Ceria to Methane. Appl. Catal. B Environ. 2002, 36, 193–206. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Yulia, V.F.; Alikina, G.M.; Lukashevich, A.I.; Muzykantov, V.S.; Rogov, V.A.; Moroz, E.M.; Zyuzin, D.A.; Ivanov, V.P.; Borchert, H.; et al. Mobility and Reactivity of Lattice Oxygen in Gd-Doped Ceria Promoted by Pt. React. Kinet. Catal. Lett. 2005, 85, 367–374. [Google Scholar] [CrossRef]
- Wei, J.; Iglesia, E. Isotopic and Kinetic Assessment of the Mechanism of Reactions of CH 4 with CO2 or H2O to Form Synthesis Gas and Carbon on Nickel Catalysts. J. Catal. 2004, 224, 370–383. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S. Promotional Effect of Gd over Ni/Y2O3 Catalyst Used in Dry Reforming of CH4 for H2 Production. Int. J. Hydrog. Energy 2017, 42, 18805–18816. [Google Scholar] [CrossRef]
- Rabelo-Neto, R.C.; Sales, H.B.E.; Inocêncio, C.V.M.; Varga, E.; Oszko, A.; Erdohelyi, A.; Noronha, F.B.; Mattos, L.V. CO2 Reforming of Methane over Supported LaNiO3 Perovskite-Type Oxides. Appl. Catal. B Environ. 2018, 221, 349–361. [Google Scholar] [CrossRef]
- Kitiyanan, B.; Alvarez, W.E.; Harwell, J.H.; Resasco, D.E. Controlled Production of Single-Wall Carbon Nanotubes by Catalytic Decomposition of CO on Bimetallic Co–Mo Catalysts. Chem. Phys. Lett. 2000, 317, 497–503. [Google Scholar] [CrossRef]
- Bonura, G.; Cannilla, C.; Frusteri, F. Ceria–Gadolinia Supported NiCu Catalyst: A Suitable System for Dry Reforming of Biogas to Feed a Solid Oxide Fuel Cell (SOFC). Appl. Catal. B Environ. 2012, 121–122, 135–147. [Google Scholar] [CrossRef]
- Taherian, Z.; Yousefpour, M.; Tajally, M.; Khoshandam, B. Promotional Effect of Samarium on the Activity and Stability of Ni-SBA-15 Catalysts in Dry Reforming of Methane. Microporous Mesoporous Mater. 2017, 251, 9–18. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.; Trimm, D.L. Mechanisms of Carbon Formation on Nickel-Containing Catalysts. J. Catal. 1977, 48, 155–165. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J. Equilibria of Decomposition Reactions of Carbon Monoxide and Methane over Nickel Catalysts. J. Catal. 1972, 27, 343–356. [Google Scholar] [CrossRef]
- Kim, J.-H.; Suh, D.J.; Park, T.-J.; Kim, K.-L. Effect of Metal Particle Size on Coking during CO2 Reforming of CH4 over Ni–Alumina Aerogel Catalysts. Appl. Catal. A Gen. 2000, 197, 191–200. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J. Coking on Nickel Catalysts for Steam Reforming of Hydrocarbons. J. Catal. 1974, 33, 184–201. [Google Scholar] [CrossRef]
- Trimm, D.L. Coke Formation and Minimisation during Steam Reforming Reactions. Catal. Today 1997, 37, 233–238. [Google Scholar] [CrossRef]
- Trimm, D.L. Catalysts for the Control of Coking during Steam Reforming. Catal. Today 1999, 49, 3–10. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R.; Sehested, J.; Nørskov, J.K. Hydrogen and Synthesis Gas by Steam- and CO2 Reforming. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 2002; Volume 47, pp. 65–139. ISBN 0360-0564. [Google Scholar]
- Bengaard, H.S.; Nørskov, J.K.; Sehested, J.; Clausen, B.S.; Nielsen, L.P.; Molenbroek, A.M.; Rostrup-Nielsen, J.R. Steam Reforming and Graphite Formation on Ni Catalysts. J. Catal. 2002, 209, 365–384. [Google Scholar] [CrossRef]
- Toebes, M.L.; Bitter, J.H.; Van Dillen, A.J.; De Jong, K.P. Impact of the Structure and Reactivity of Nickel Particles on the Catalytic Growth of Carbon Nanofibers. Catal. Today 2002, 76, 33–42. [Google Scholar] [CrossRef]
- Chen, D.; Christensen, K.O.; Ochoa-Fernández, E.; Yu, Z.; Tøtdal, B.; Latorre, N.; Monzón, A.; Holmen, A. Synthesis of Carbon Nanofibers: Effects of Ni Crystal Size during Methane Decomposition. J. Catal. 2005, 229, 82–96. [Google Scholar] [CrossRef]
- Vogt, C.; Kranenborg, J.; Monai, M.; Weckhuysen, B.M. Structure Sensitivity in Steam and Dry Methane Reforming over Nickel: Activity and Carbon Formation. ACS Catal. 2020, 10, 1428–1438. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Su, T.; Qin, Z.; Ji, H. Coke-Resistant Ni-Based Bimetallic Catalysts for the Dry Reforming of Methane: Effects of Indium on the Ni/Al2O3 Catalyst. Catal. Sci. Technol. 2022, 12, 4826–4836. [Google Scholar] [CrossRef]
- Yan, N. Ni-Ir/MgAl2O4 for Balanced Carbon Deposition-Elimination in Methane Dry Reforming. Chem Catal. 2022, 2, 1520–1521. [Google Scholar] [CrossRef]
- Lercher, J.A.; Bitter, J.H.; Hally, W.; Niessen, W.; Seshan, K. Design of Stable Catalysts for Methane-Carbon Dioxide Reforming. In Studies in Surface Science and Catalysis, Proceedings of the 11th International Congress On Catalysis—40th Anniversary, Baltimore, MD, USA, 30 June–5 July 1996; Hightower, J.W., Nicholas Delgass, W., Iglesia, E., Bell, A.T., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 101, pp. 463–472. [Google Scholar]
- Baktash, E.; Littlewood, P.; Schomäcker, R.; Thomas, A.; Stair, P.C. Alumina Coated Nickel Nanoparticles as a Highly Active Catalyst for Dry Reforming of Methane. Appl. Catal. B Environ. 2015, 179, 122–127. [Google Scholar] [CrossRef]
- Han, B.; Wang, F.; Zhang, L.; Wang, Y.; Fan, W.; Xu, L.; Yu, H.; Li, Z. Syngas Production from Methane Steam Reforming and Dry Reforming Reactions over Sintering-Resistant Ni@SiO2 Catalyst. Res. Chem. Intermed. 2019, 46, 1735–1748. [Google Scholar] [CrossRef]
- Huang, Q.; Fang, X.; Cheng, Q.; Li, Q.; Xu, X.; Xu, L.; Liu, W.; Gao, Z.; Zhou, W.; Wang, X. Synthesis of a Highly Active and Stable Nickel-Embedded Alumina Catalyst for Methane Dry Reforming: On the Confinement Effects of Alumina Shells for Nickel Nanoparticles. ChemCatChem 2017, 9, 3563–3571. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, F.; Zhu, J.; Han, B.; Fan, W.; Zhao, L.; Cai, W.; Li, Z.; Xu, L.; Yu, H.; et al. CO2 Reforming with Methane Reaction over Ni@SiO2 Catalysts Coupled by Size Effect and Metal-Support Interaction. Fuel 2019, 256, 115954. [Google Scholar] [CrossRef]
- Ross, J.R.H.; Van Keulen, A.N.J.; Hegarty, M.E.S.; Seshan, K. The Catalytic Conversion of Natural Gas to Useful Products. Catal. Today 1996, 30, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Laosiripojana, N.; Assabumrungrat, S. Catalytic Dry Reforming of Methane over High Surface Area Ceria. Appl. Catal. B Environ. 2005, 60, 107–116. [Google Scholar] [CrossRef]
- Vlaic, G.; Fornasiero, P.; Geremia, S.; Kašpar, J.; Graziani, M. Relationship between the Zirconia-Promoted Reduction in the Rh-Loaded Ce0.5Zr0.5O2Mixed Oxide and the Zr–O Local Structure. J. Catal. 1997, 168, 386–392. [Google Scholar] [CrossRef]
- Huang, T.-J.; Lin, H.-J.; Yu, T.-C. A Comparison of Oxygen-Vacancy Effect on Activity Behaviors of Carbon Dioxide and Steam Reforming of Methane over Supported Nickel Catalysts. Catal. Lett. 2005, 105, 239–247. [Google Scholar] [CrossRef]













| Catalyst | Ni (wt%) | CeO2 (wt%) | Dopant (wt%) | Ce/Dopant Molar Ratio | Surface Area of Reduced Sample (m2/g) |
|---|---|---|---|---|---|
| Ni@CeO2 | 9.7 | 87.2 | --- | --- | <10 |
| Ni@CeGdO2 | 9.8 | 72.1 | 15.1 | 4.2 | <10 |
| Ni@CeSmO2 | 9.7 | 72.4 | 17.9 | 4.1 | <10 |
| Ni@CeZrO2 | 9.5 | 74.4 | 13.4 | 4.0 | 20 |
| Catalyst | Ni Crystallite Size (nm) | TOF (s−1) |
|---|---|---|
| Ni@CeO2 | 10.7 | 3.6 |
| Ni@CeGdO2 | 11.0 | 3.6 |
| Ni@CeSmO2 | 9.5 | 3.7 |
| Ni@CeZrO2 | 5.4 | 2.7 |
| Catalyst | H2 Uptake (μmol/g) | Theoretical H2 Consumption for Total Reduction (μmol/g) | Ce4+/Ce3+ Reduction (%) | ||
|---|---|---|---|---|---|
| Low Temperature | High Temperature | Ni2+/Ni0 | Ce4+/Ce3+ | ||
| Ni@CeO2 | 2022.7 | 643.3 | 1652.7 | 5066.4 | 20 |
| Ni@CeGdO2 | 1963.0 | ----- | 1669.7 | 4190.1 | 7 |
| Ni@CeSmO2 | 2199.7 | ----- | 1652.7 | 4207.5 | 13 |
| Ni@CeZrO2 | 3131.4 | ----- | 1618.5 | 4322.7 | 35 |
| Catalyst | Reaction Conditions | Rate of Carbon Formation (mgC·gcat−1·h−1) | Reference |
|---|---|---|---|
| Ni@CeO2 | 800 °C, CH4:CO2 = 1:1 | 1.6 | This work |
| Ni@CeGdO2 | 800 °C, CH4:CO2 = 1:1 | 21.9 | This work |
| Ni@CeSmO2 | 800 °C, CH4:CO2 = 1:1 | 9.8 | This work |
| Ni@CeZrO2 | 800 °C, CH4:CO2 = 1:1 | 0.0 | This work |
| Ni/CeO2 | 800 °C, CH4:CO2 = 1:1 | 9.7 | [6] |
| Ni/CeO2 | 700 °C, CH4:CO2 = 1:1 | 0.4 | [34] |
| Ni/CeZrO2 (75 wt% CeO2) | 700 °C, CH4:CO2 = 1:1 | 3.5 | [34] |
| Ni/CeZrO2 (44 wt% CeO2) | 700 °C, CH4:CO2 = 1:1 | 1.7 | [34] |
| Ni/ CeZrO2 (28 wt% CeO2) | 700 °C, CH4:CO2 = 1:1 | 0.7 | [34] |
| NiCu/Ce0.9Gd0.1O2 | 800 °C, CH4:CO2 = 1:1 | 12.2 | [60] |
| LaNiO3 | 800 °C, CH4:CO2 = 1:1 | 27.0 | [58] |
| LaNiO3/SiCeO2 | 800 °C, CH4:CO2 = 1:1 | 0.3 | [58] |
| Ni/Gd-Y2O3 (1% Gd) | 700 °C, CH4:CO2 = 1:1 | 17.0 | [57] |
| Ni/Gd-Y2O3 (2% Gd) | 700 °C, CH4:CO2 = 1:1 | 14.6 | [57] |
| Ni/Gd-Y2O3 (3% Gd) | 700 °C, CH4:CO2 = 1:1 | 11.8 | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinho, A.L.A.; Rabelo-Neto, R.C.; Epron, F.; Toniolo, F.S.; Noronha, F.B.; Bion, N. Effect of Metal Dopant on the Performance of Ni@CeMeO2 Embedded Catalysts (Me = Gd, Sm and Zr) for Dry Reforming of Methane. Methane 2022, 1, 300-319. https://doi.org/10.3390/methane1040023
Marinho ALA, Rabelo-Neto RC, Epron F, Toniolo FS, Noronha FB, Bion N. Effect of Metal Dopant on the Performance of Ni@CeMeO2 Embedded Catalysts (Me = Gd, Sm and Zr) for Dry Reforming of Methane. Methane. 2022; 1(4):300-319. https://doi.org/10.3390/methane1040023
Chicago/Turabian StyleMarinho, André L. A., Raimundo C. Rabelo-Neto, Florence Epron, Fabio S. Toniolo, Fabio B. Noronha, and Nicolas Bion. 2022. "Effect of Metal Dopant on the Performance of Ni@CeMeO2 Embedded Catalysts (Me = Gd, Sm and Zr) for Dry Reforming of Methane" Methane 1, no. 4: 300-319. https://doi.org/10.3390/methane1040023
APA StyleMarinho, A. L. A., Rabelo-Neto, R. C., Epron, F., Toniolo, F. S., Noronha, F. B., & Bion, N. (2022). Effect of Metal Dopant on the Performance of Ni@CeMeO2 Embedded Catalysts (Me = Gd, Sm and Zr) for Dry Reforming of Methane. Methane, 1(4), 300-319. https://doi.org/10.3390/methane1040023