Recent Advances in Protein Phosphorylation (Closed)
A topical collection in Biomolecules (ISSN 2218-273X). This collection belongs to the section "Molecular Biology".
Viewed by 22577Editors
Interests: phosphoproteomics; posttranslational modification; protein kinase; Phos-tag
Interests: protein phosphorylation; two-component signal transduction system; histidine kinase; Phos-tag
Topical Collection Information
Dear Colleagues,
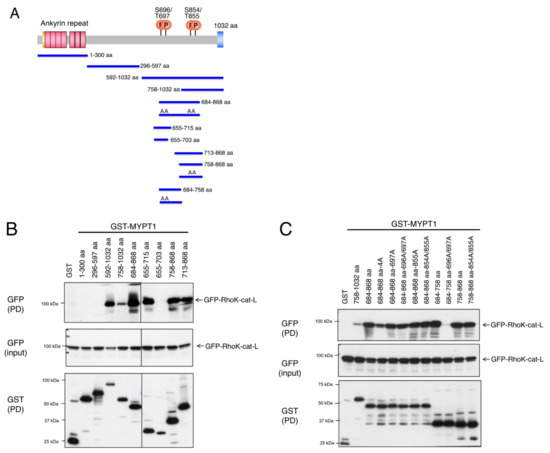
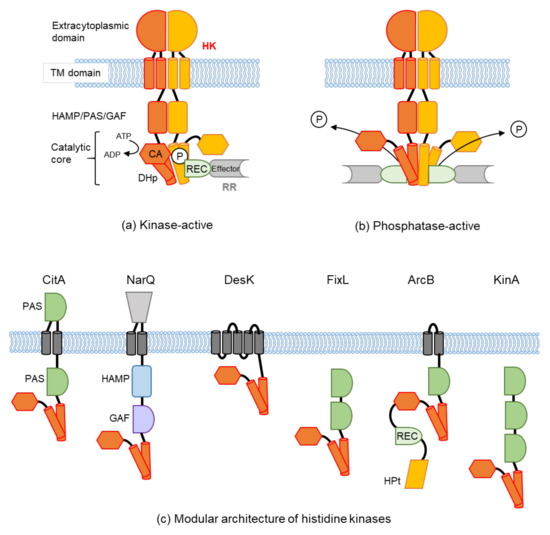
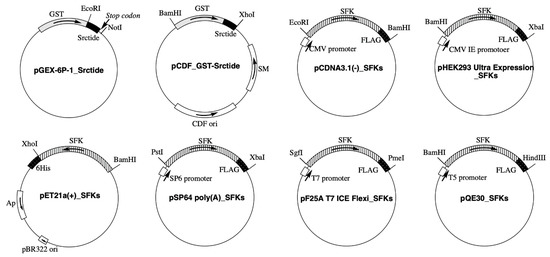
Protein phosphorylation is a common type of posttranslational modification (PTM) that is present in all biological species, and it affects key properties of proteins involved in numerous cellular events. In living cells, the continuous and dynamic phosphorylation and dephosphorylation of proteins at specific amino acid residues are controlled by complex signaling networks, resulting in the production of a variety of phosphoproteins with various states of phosphorylation. Protein phosphorylation occurs on several amino acid residues, including His, Asp, Glu, Lys, Arg, and Cys, on which it is very labile and difficult to detect, while more stable phosphorylation takes place on three specific residues, Ser, Thr, and Tyr. In bacterial cells, His- and Asp-phosphorylated proteins are well known for their leading roles in the two-component signal transduction system. In higher eukaryotic cells, on the other hand, Ser-, Thr-, and Tyr-phosphorylated proteins are predominant. Since the standard free energies for bonds of imidazole-phosphate on the His residue and carboxyl-phosphate on the Asp residue are large, the phosphorylated His and Asp have the potential to serve as intermediates in phosphotransfer reactions to other amino acids. Therefore, in addition to Ser-, Thr-, and Tyr-phosphorylated proteins, His- and Asp-phosphorylated proteins play crucial roles as a sensor apparatus and a response regulator, respectively, of the two-component system in quick response to intra- and extracellular signals in prokaryotes as well as in fungi and plants.
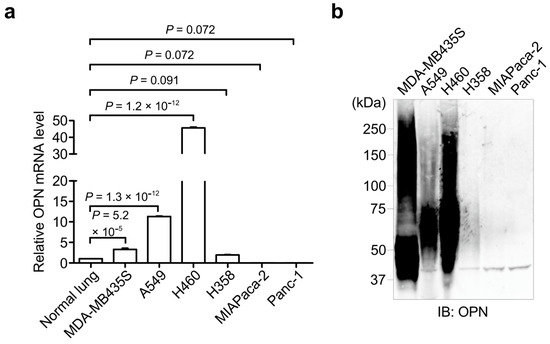
This reversible PTM is generally catalyzed by the opposing activities of large families of protein kinase and phosphatase enzymes. For example, the human genome encodes more than 500 protein kinases and about 300 protein phosphatases. Approximately 13000 human proteins have sites that are phosphorylated and dephosphorylated. These numbers reflect the importance and the complexity of protein phosphorylation. Abnormal phosphorylation resulting from an imbalance in the enzymatic reactions of kinases and phosphatases has been implicated in a wide range of human diseases, including cancer, diabetes mellitus, neurodegeneration, and immune/inflammatory and vascular disorders. Therefore, methods for quantitative and qualitative monitoring of alterations in the phosphorylation states of certain proteins are also very important for studies on the proteome, particularly in relation to the elucidation of the molecular origins of diseases and the rational molecular design of drugs.
This Special Issue will focus on the role of protein phosphorylation in all living cells. Original manuscripts and reviews dealing with any aspect of protein phosphorylation and related pathophysiology and methodology are very welcome.
Dr. Eiji Kinoshita
Dr. Emiko Kinoshita-Kikuta
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Biomolecules is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- protein phosphorylation
- protein kinase
- protein phosphatase
- phosphoproteomics