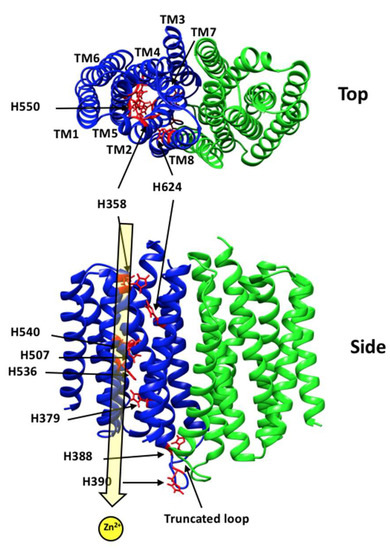
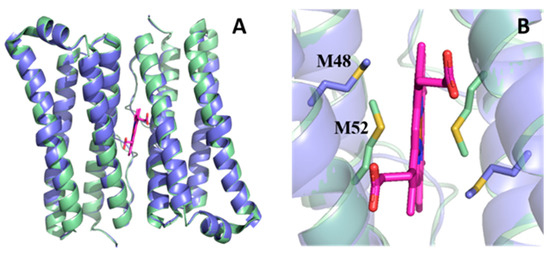
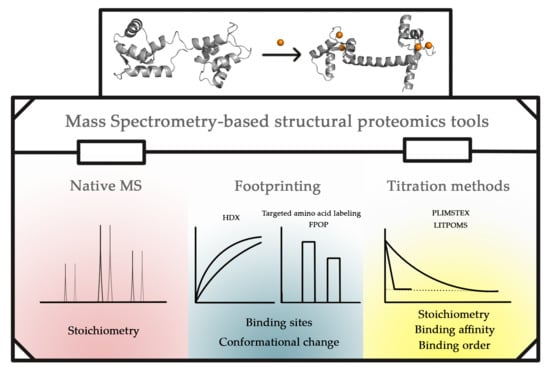
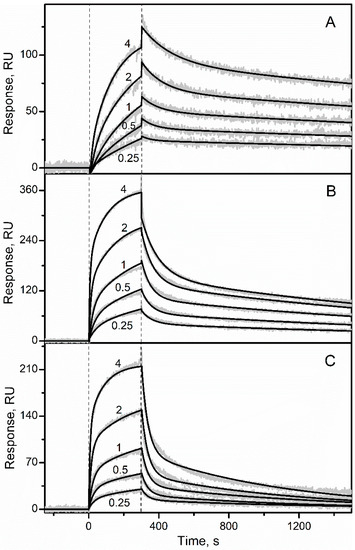
Advances in Metal Binding Proteins
A topical collection in Biomolecules (ISSN 2218-273X). This collection belongs to the section "Cellular Biochemistry".
Viewed by 34775Editor
Interests: protein physics; luminescence (fluorescence, phosphorescence) spectroscopy of proteins; scanning and titration calorimetry of proteins; metal binding proteins; calcium binding proteins
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Everyone knows that the functioning of any biological system is impossible without metal ions. The ‘metals of life’ include sodium, potassium, magnesium, calcium, manganese, iron, cobalt, zinc, nickel, vanadium, molybdenum, and tungsten. Any biological system contains special proteins specifically interacting with metal ions. Metal ions play several major roles in proteins: structural, regulatory, and enzymatic. Strong binding of some metal ions increases the stability of proteins or protein domains. Some metal ions can regulate various cell processes being first, second, or third messengers. Calcium is the most universal carrier of signals to cells. It regulates all important aspects of cell activity, beginning with fertilization and ending with apoptotic suicide at the end of the life cycle. Metal ions are an essential part of many enzymes and are indispensable in many catalytic reactions.
Despite the apparent good knowledge of the structure and properties of many metal-binding proteins, many aspects of their structure and, in particular, mechanisms of their functioning are still insufficiently studied. For example, although the three-dimensional structure of many calcium-binding sites in proteins is well known, a closer look at their environment reveals common structural features in these proteins that were not previously noticed. Studying the interactions of metal binding proteins with proteins of other classes allows us to reach a better understanding of their physiological functions. Modern methods of genetic engineering and knowledge of the three-dimensional structure of metal-binding proteins allow us to study their structure and functions at a new level. The obtained fundamental knowledge can already be used in applied science. Comprehensive reviews or original research articles are most welcome.
Dr. Eugene Permyakov
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Biomolecules is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- metal ions
- metal binding proteins
- regulation
- enzymes
- structure
- function
Planned Papers
The below list represents only planned manuscripts. Some of these manuscripts have not been received by the Editorial Office yet. Papers submitted to MDPI journals are subject to peer-review.
Title: Regulation of FYVE domain interactions with membranes
Authors: Cameron Smithers; Troy Kervin; Michael Overduin
Affiliation: University of Alberta, Edmonton, Canada
Title: To be determined
Authors: Svetlana Lutsenko; Jenifer Calvo; Abigael Muchenditsi; et al.
Affiliation: Johns Hopkins University, USA
Title: To be determined
Authors: Artem Bonchuk; et al.
Affiliation: Department of the Control of Genetic Processes, Institute of Gene Biology, Russian Academy of Sciences, 3 4/5 Vavilov St., Moscow, 119334, Russia