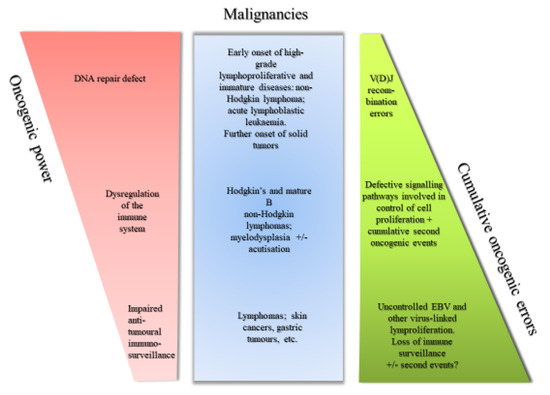
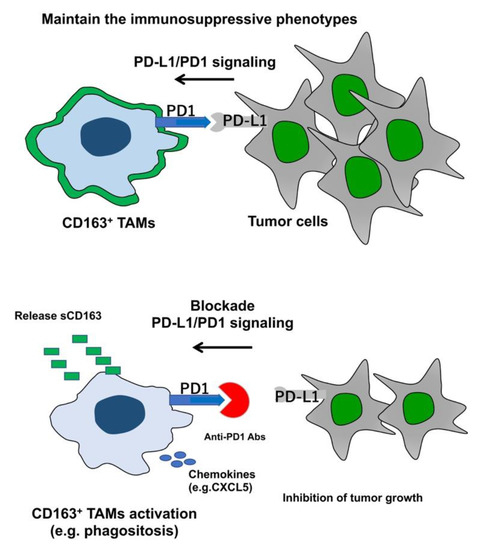
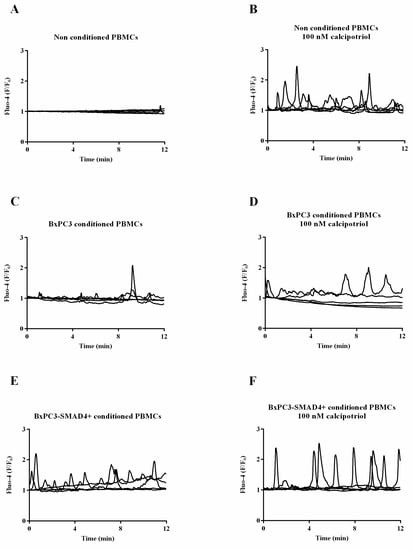
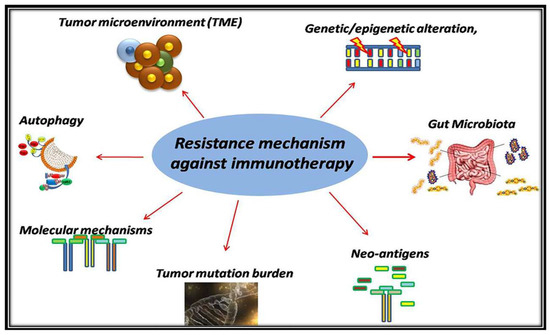
Recent Advances in Cancer Immunotherapy
A topical collection in Biomolecules (ISSN 2218-273X). This collection belongs to the section "Molecular Medicine".
Viewed by 41247Editor
Interests: lung cancer; tumor heterogeneity; inherent and acquired resistance mechanisms; EGFR mutation; biomarkers; immunotherapy
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Cancer immunotherapy has introduced a novel therapeutic concept in oncology: eliminating cancer cells via the activation of the immune system but not directly attacking cancer cells. Cancer immunotherapy, immune checkpoint inhibitors (ICIs), and chimeric antigen receptor (CAR) T cell therapy have now become standard treatment methods for a broad variety of hematological or solid malignancies. It is noteworthy that, cancer immunotherapy sometimes induces durable long-term efficacy in certain patients, offering hope for a cure in those individuals. In addition, to improve treatment outcomes of cancer immunotherapy further, numerous clinical trials are now ongoing worldwide. In this Topical Collection, we aim to publish recent advances in cancer immunotherapy from various perspectives, including basic research, translational data, clinical observations, and clinical trials, as well as comprehensive reviews that focus on cancer immunotherapy in malignancies such as melanoma, lung cancer, urothelial cancer, renal cell carcinoma, head and neck squamous cell carcinoma, and hematological malignancies.
This Topical Collection will cover all aspects of recent advances in cancer immunotherapies, including ICIs and CAR T cell therapies, in hematological and solid malignancies. This Topical Collection will include review articles (especially with respect to biomarkers for immunotherapies, resistance mechanisms to immunotherapies, or novel immune-related targets), original research articles in this field, and short contributions. This Topical Collection will also accept case series if the treatment course of the patient is educational or the detailed molecular analysis was performed to investigate resistance mechanisms to cancer immunotherapy.
Dr. Kenichi SudaCollection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Biomolecules is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- immune checkpoint inhibitors (ICIs)
- chimeric antigen receptor (CAR) T cell
- predictive biomarkers
- resistance mechanisms
- combination approaches
- toxicity management
- immunotherapies in neoadjuvant/adjuvant settings
- novel targets of immunotherapies
- tumor microenvironment