Cell-to-Cell Metabolic Cross-Talk in Physiology and Pathology (Closed)
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cell Microenvironment".
Viewed by 40012Editors
Interests: autophagy and protein metabolism in cancer and in neurodegeneration; epigenetics in cancer; nutraceuticals and probiotics in cancer and infectious diseases
Special Issues, Collections and Topics in MDPI journals
Interests: cancer; cell signaling; non-coding RNA; tumor microenvironment
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
This Special Issue of Cells focuses on the mechanisms and pathophysiological role of the metabolic crosstalk between cells and includes selected papers from invited speakers and registered participants of the 1st electronic Cells 2020 conference on “Cell-to-Cell Metabolic Crosstalk in Physiology and Pathology” (https://sciforum.net/conference/Cells2020).
Submitted papers will be subjected to peer review and are published with the aim of rapid and wide dissemination of research results, developments, and applications.
The conference will be held from 17 December 2020 to 17 January 2021 on sciforum.net, a platform service for hosting international electronic conferences for scientific communities. Lectures will be presented live through a series of webinars.
The topical sessions include:
- Cell Cycle Regulators: The Crosstalk with Metabolism
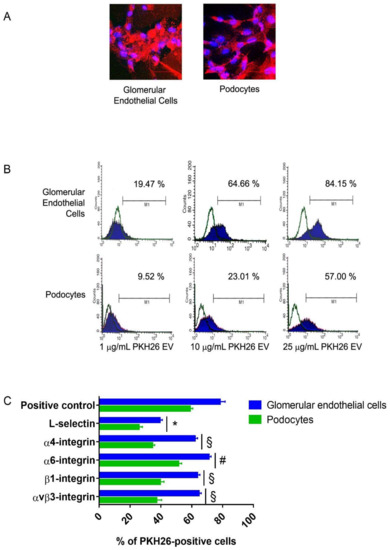
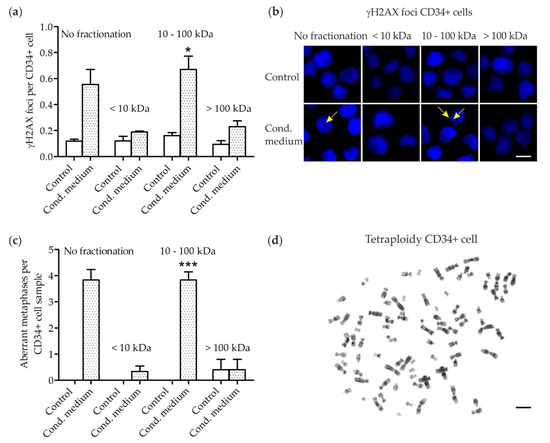
- Exosomes and Extracellular Vesicles in Health and Disease
- The Crosstalk between Cell Adhesion and Metabolism
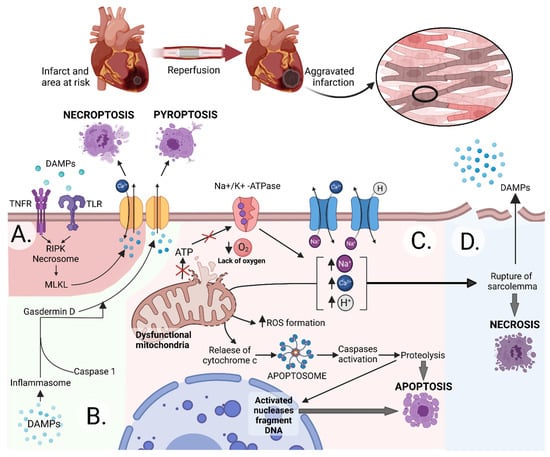
- Crosstalk between Cell Death Regulation and Metabolism
- Crosstalk between Immune Cells and Tissue Microenvironment
- Compartmentalization of Cellular Signaling
Prof. Dr. Ciro Isidoro
Prof. Dr. Danny D Dhanasekaran
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- exosomes
- cell death
- cell metabolism
- diseases
- autophagy
- cell adhesion
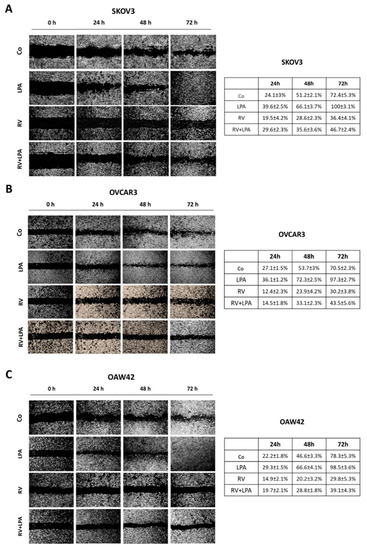
- cell migration
- cell signaling
- cell proliferation
- tissue microenvironment