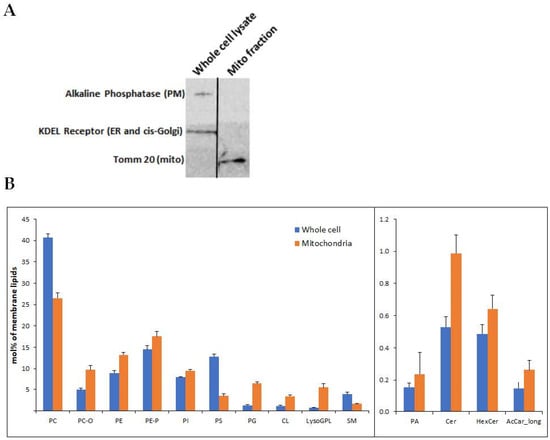
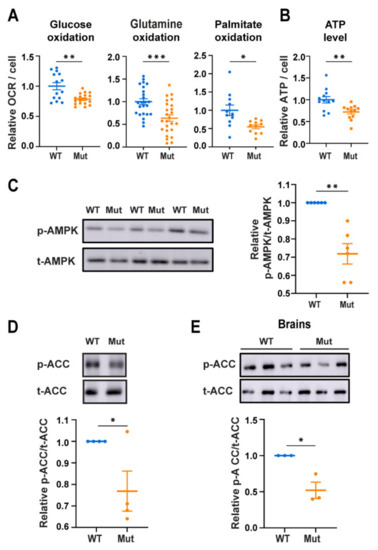
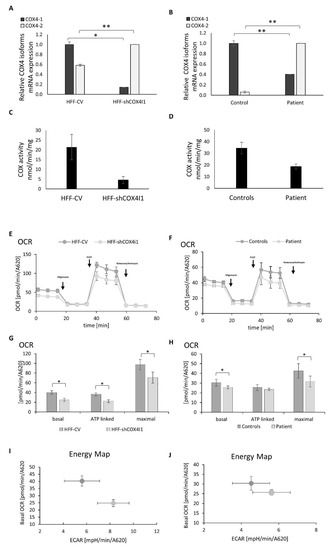
The Pathomechanism of Mitochondrial Diseases
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Mitochondria".
Viewed by 33583Editor
Interests: pathomechanism of inborn errors of metabolism and mitochondrial diseases; mitochondrial respiratory chain function in health and disease
Topical Collection Information
Dear Colleagues,
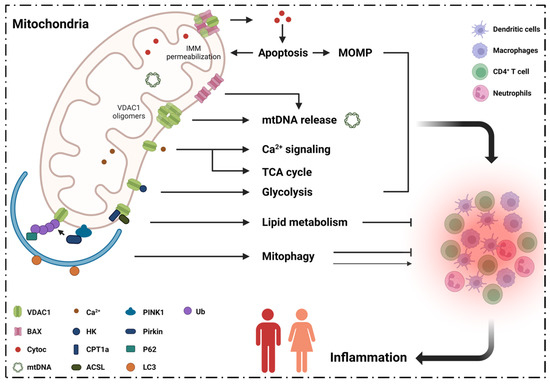
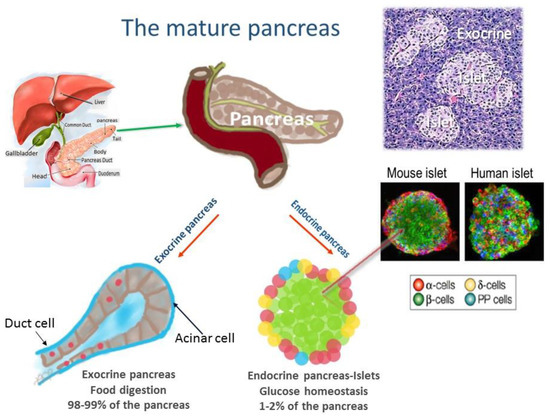
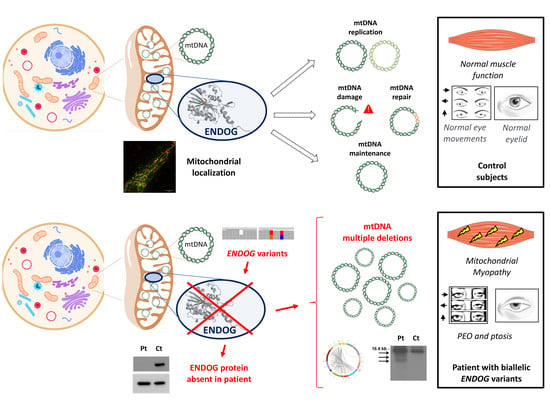
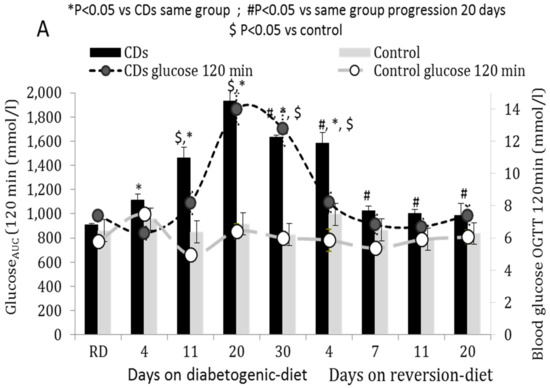
Mitochondrial diseases are a heterogenous group of common inborn errors of metabolism, originating from pathogenic variants in the mitochondrial or nuclear genome, resulting in respiratory chain deficiency, mostly with devastating consequences. Moreover, mitochondrial dysfunction is associated with numerous other pathological conditions, including neurodegenerative diseases, diabetes, cancer, immune dysfunction, and heart and kidney diseases.
Although next-generation sequencing (NGS) techniques are a game changer in the diagnosis of mitochondrial diseases, they have not signtificantly resulted in providing better treatment options, which are presently very limited. Studying the patomechanistic aspects (cell biology and physiology, molecular biology, and biophysics) of the individual diseases in cellular and animal models is a vital step towards developing more effective treatments.
The aim of this Topical Collection is to broaden knowledge and understanding of the various pathomechanisms involving mitochondrial dysfuction in disease, including evaluating the effect of small molecules and other treatments in model systems.
Prof. Ann Saada
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- mitochondrial diseases
- mitochondrial dysfunction
- mitochondrial respiratory chain
- mitochondrial DNA
- pathomechanism
- treatment