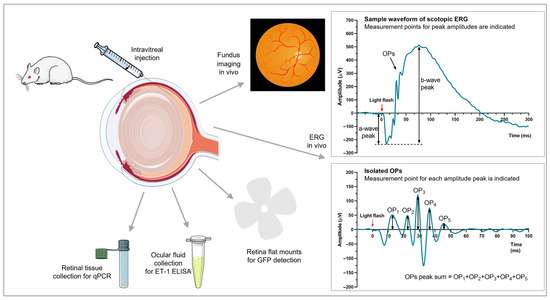
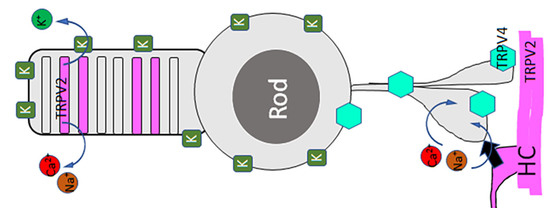
Animal Models of Retinal Degeneration
A topical collection in Cells (ISSN 2073-4409).
Viewed by 13314Editor
Interests: photoreceptors; cyclic nucleotides; retinal degeneration; animal models; molecular neuroscience
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
The retina is an ideal CNS tissue to study because of its accessibility and the availability of many well-characterized animal models that exhibit ocular pathologies mimicking diseases seen in patients. The first successful gene therapy in the U.S. approved for use in patients was to treat a hereditary retinal degeneration called Leber congenital amaurosis, an early-onset form of the family of diseases collectively called retinitis pigmentosa. That pioneering achievement was based upon years of experimental studies using animal models of the human disease. There is still much to be done, and the recent development of new research tools for gene editing and other genome manipulations promises to bring an era of even greater accomplishments in the study and treatment of retinal diseases. With this new thematic collection, we aim to attract the most promising research in the field to drive further advancements that will ultimately lead to curative life-long interventions for the many hundreds of ocular disorders already known, as well as those yet to be discovered.
Prof. Steven J. Pittler
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- retinitis pigmentosa
- age-related macular degeneration
- amphibians
- rodents
- primates
- gene editing
- translational
- transgenesis
- review
- transplantation
- regeneration
- clinical correlation