Journal Description
Pharmacoepidemiology
Pharmacoepidemiology
is an international, peer-reviewed, open access journal on high-quality epidemiological, clinical research across the fields of clinical pharmacology and epidemiology, published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 29.5 days after submission; acceptance to publication is undertaken in 4.7 days (median values for papers published in this journal in the second half of 2024).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
- Pharmacoepidemiology is a companion journal of Pharmaceuticals and Journal of Clinical Medicine.
Latest Articles
A Systematic Review of Potential Opioid Prescribing Safety Indicators
Pharmacoepidemiology 2025, 4(1), 4; https://doi.org/10.3390/pharma4010004 - 8 Jan 2025
Abstract
►
Show Figures
Background/Objectives: This systematic review aimed to identify a comprehensive list of potential opioid-related indicators from the published literature to assess prescribing safety in any setting. Methods: Studies that reported prescribing indicators from 1990 to 2019 were retrieved from a previously published systematic review.
[...] Read more.
Background/Objectives: This systematic review aimed to identify a comprehensive list of potential opioid-related indicators from the published literature to assess prescribing safety in any setting. Methods: Studies that reported prescribing indicators from 1990 to 2019 were retrieved from a previously published systematic review. A subsequent search was conducted from seven electronic databases to identify additional studies from 2019 to June 2024. Potential opioid safety prescribing indicators were extracted from studies that reported prescribing indicators of non-injectable opioids prescribed to adults with concerns about the potential risk of harm. The retrieved indicators were split by each opioid, and duplicates were removed. The identified indicators were categorized by the type of problem, medication, patient condition/disease, and the risk of the indicators. Results: A total of 99 unique opioid-specific prescribing indicators were identified from 53 included articles. Overall, 42 (42%) opioid prescribing indicators focused on a specific class of opioids. Pethidine, tramadol, and fentanyl were the most frequently reported drugs (n = 22, 22%). The indicators account for six types of problems: medication inappropriate for the population (n = 20), omission (n = 8), inappropriate duration (n = 10), inadequate monitoring (n = 2), drug–disease interaction (n = 26), and drug–drug interaction (n = 33). Of all the indicators, older age (over 65) is the most common risk factor (n = 38, 39%). Central nervous system-related adverse effects are the risk of concern for the 28 (29%) indicators associated with drug–drug interactions. Furthermore, five of the six ’omission’ indicators are related to ’without using laxatives’. Conclusions: This review identified a comprehensive set of indicators for flagging patients at high risk of opioid-related harm, thereby supporting informed decision-making in optimizing opioid utilization. However, further research is essential to validate these indicators and evaluate their feasibility across diverse healthcare settings.
Full article
Open AccessArticle
Antibiotic Consumption Patterns in Urban Coari, Amazonas: High Azithromycin Use and COVID-19-Related Prescriptions
by
Rodrigo Silva Marcelino, Edivã Bernardo da Silva, Abel Santiago Muri Gama, Ananias Facundes Guimarães, Silvia Regina Secoli and Albert Figueras
Pharmacoepidemiology 2025, 4(1), 3; https://doi.org/10.3390/pharma4010003 - 4 Jan 2025
Abstract
►▼
Show Figures
Background/Objectivses: Antibiotic consumption patterns in remote urban areas of the Amazon region are poorly understood. This study aimed to analyze antibiotic use in the adult population of Coari, a municipality in Amazonas, Brazil. Methods: A cross-sectional study was conducted between October
[...] Read more.
Background/Objectivses: Antibiotic consumption patterns in remote urban areas of the Amazon region are poorly understood. This study aimed to analyze antibiotic use in the adult population of Coari, a municipality in Amazonas, Brazil. Methods: A cross-sectional study was conducted between October and November 2021 in the urban area of Coari. 394 adults were interviewed using a structured questionnaire. Data on antibiotic use, sociodemographic factors, health service access, and self-reported illnesses were collected. Poisson regression was used to estimate prevalence ratios and identify factors associated with antibiotic use. Results: The prevalence of antibiotic use was 14.7% (n = 58). The most frequently used antibiotics were azithromycin (26.9%), cefalexin (20.9%), amoxicillin (19.4%), and ciprofloxacin (13.9%). Up to 34.5% of antibiotic use was conducted without a prescription, especially among adults aged 18 to 39 (59.1%). The main health problems that led to self-medication were COVID-19 (28.6%), urinary infection (14.3%), sore throat (37.5%), and intestinal infection (60.0%). Factors associated with antibiotic use included age 18 to 39 (adjusted PR = 3.73; CI = 1.37–10.09), having a family member hospitalized (adjusted PR = 2.61; CI = 1.39–4.89), having contracted COVID-19 (adjusted PR = 2.41; CI = 1.40–4.15), and frequency of visits by the community health agent to the home (adjusted PR = 0.35 CI = 0.15–0.81). Conclusions: The high use of broad-spectrum antibiotics (Watch), particularly azithromycin, for potentially inappropriate indications highlights the need to improve the management of antibiotic use in remote regions of Brazil. Community health agents, as key professionals between health services and the community, can play a key role in promoting the rational use of antibiotics and combating antimicrobial resistance in the Brazilian Amazon context.
Full article

Figure 1
Open AccessArticle
Cost-Effectiveness of Itopride Hydrochloride for the Treatment of Functional Dyspepsia in Vietnam
by
Hansoo Kim, Joshua Byrnes, Kyoo Kim, Duc Trong Quach, Tran Thi Khanh Tuong and Cuc Thi Thu Nguyen
Pharmacoepidemiology 2025, 4(1), 2; https://doi.org/10.3390/pharma4010002 - 3 Jan 2025
Abstract
►▼
Show Figures
Background/Objectives: Functional dyspepsia is associated with abdominal pain and nausea, which leads to reduced quality of life, loss of productivity, and economic loss for patients. Itopride hydrochloride (itopride) stimulates the gastrointestinal smooth muscles, thereby promoting gastric emptying. It has been shown to significantly
[...] Read more.
Background/Objectives: Functional dyspepsia is associated with abdominal pain and nausea, which leads to reduced quality of life, loss of productivity, and economic loss for patients. Itopride hydrochloride (itopride) stimulates the gastrointestinal smooth muscles, thereby promoting gastric emptying. It has been shown to significantly improve symptoms in patients with functional dyspepsia without severe side effects. Itopride has been available in Vietnam for many years; however, the cost-effectiveness of the drug has not been established. The aim of this study is to estimate the cost-effectiveness of itopride for the treatment of functional dyspepsia in Vietnam. Methods: A 3-stage Markov model with the following health states—controlled functional dyspepsia, uncontrolled functional dyspepsia, and dead—was developed. Functional dyspepsia was used to assess itopride over 10 years using 8-week cycles. A broader Vietnamese societal perspective was assumed for the analysis. Input was retrieved from the literature and through local clinical input from physicians in Vietnam. Output was reported as an incremental cost-effectiveness ratio (ICER) per quality-adjusted life years (QALY). A GDP/capita threshold (very cost-effective: 1 × GDP = Vietnamese Dong (VND) 64.1 M, cost-effective: 3 × GDP = VND 192.2 M) was used as recommended by the WHO in Vietnam. One-way and probabilistic sensitivity analyses were performed. Results: Itopride use resulted in an additional 0.28 QALYs at an extra cost of VND 11.2 M. This resulted in an ICER of VND 39.7 M per QALY, which is lower than the threshold of VND 192.2 M. One-way sensitivity analyses showed that the ICER was sensitive to varying the efficacy VND 31.8 M to VND 88.3 M), cost of itopride (ICER: VND 43.1 M to VND 56.5 M), and the health utility values (ICER: VND 45.2 M to VND 55.3 M). More than 80% of the simulations in the probabilistic sensitivity analysis were cost-effective at the 1 × GDP (VND 64.1 M) threshold, and 91.3% were cost-effective at the 3 × GDP (VND 192.2 M) threshold. Conclusion: This study shows that itopride hydrochloride is a very cost-effective treatment for functional dyspepsia in Vietnam, with the ICER (VND 39.7 M/QALY) being even lower than the 1 × GDP (VND 64.1 M) threshold.
Full article

Figure 1
Open AccessArticle
Hepatobiliary Adverse Events Associated with Pembrolizumab: A Pharmacovigilance Study from the FDA Adverse Event Reporting System (FAERS) Database
by
Connor Frey
Pharmacoepidemiology 2025, 4(1), 1; https://doi.org/10.3390/pharma4010001 - 30 Dec 2024
Abstract
►▼
Show Figures
Background: Immuno-oncology has transformed cancer treatment, with immune checkpoint inhibitors (ICIs) like pembrolizumab playing a key role. While highly effective, these therapies can also cause immune-related adverse events. This study examines the incidence and characteristics of hepatobiliary adverse events (AEs) linked to pembrolizumab,
[...] Read more.
Background: Immuno-oncology has transformed cancer treatment, with immune checkpoint inhibitors (ICIs) like pembrolizumab playing a key role. While highly effective, these therapies can also cause immune-related adverse events. This study examines the incidence and characteristics of hepatobiliary adverse events (AEs) linked to pembrolizumab, using data from the FDA Adverse Event Reporting System (FAERS). Objective: To investigate the rates of hepatobiliary AEs linked to pembrolizumab, providing insights into the risks of liver and biliary system damage in patients prescribed pembrolizumab. Methods: This study utilized the FAERS database via OpenVigil 2.1. Adverse events (AEs) related to pembrolizumab were identified and compared to those associated with other drugs. Reporting odds ratios (RORs) were calculated to assess the likelihood of hepatobiliary AEs in pembrolizumab-treated patients. Results: In total, 594 hepatic AEs and 181 biliary AEs were identified. Significant hepatic AEs included elevated ALT (ROR 3.00, 95% CI: 2.685–3.351), hepatotoxicity (ROR 6.436, 95% CI: 5.72–7.242), and hepatic cytolysis (ROR 15.721, 95% CI: 13.854–17.84). Immune-mediated hepatitis exhibited the highest ROR of 346.716 (95% CI: 303.568–395.997). For biliary AEs, cholangitis (ROR 19.597, 95% CI: 16.852–22.791) and sclerosing cholangitis (ROR 24.735, 95% CI: 19.888–30.763) were the most prominent. Conclusions: Pembrolizumab is associated with a significant risk of hepatobiliary adverse events, particularly immune-mediated hepatitis and cholangitis. The elevated RORs for these conditions highlight the importance of close monitoring and managing liver and biliary functions in patients undergoing pembrolizumab checkpoint blockade. These findings emphasize the need for personalized treatment strategies to mitigate risks and optimize outcomes in cancer immunotherapy, especially for those with preexisting hepatobiliary conditions.
Full article

Graphical abstract
Open AccessCase Report
Fixed Drug Eruption Due to Doxycycline Postexposure Prophylaxis
by
Lee Nguyen and Catherine Diamond
Pharmacoepidemiology 2024, 3(4), 394-402; https://doi.org/10.3390/pharma3040028 - 11 Dec 2024
Abstract
►▼
Show Figures
Doxycycline is a semi-synthetic antibiotic in the tetracycline family. The three common subtypes of tetracyclines include naturally occurring, semi-synthetic, and new agents. Each subtype shares specific commonalities but is substantially different in various clinical applications. The mechanism of antimicrobial activity is the same
[...] Read more.
Doxycycline is a semi-synthetic antibiotic in the tetracycline family. The three common subtypes of tetracyclines include naturally occurring, semi-synthetic, and new agents. Each subtype shares specific commonalities but is substantially different in various clinical applications. The mechanism of antimicrobial activity is the same across subtypes. The structural changes to the core naphthacene ring do not alter the mechanism of action but are thought to alter the rates of adverse effects and mechanisms of resistance. Tetracyclines as a class are known to cause fixed drug eruptions, but the majority of these adverse effects were associated with naturally occurring tetracyclines. Semi-synthetic tetracyclines have limited reports of fixed drug eruptions. Here, we present a case of fixed drug eruption in a patient who previously had multiple treatment courses with doxycycline. The case involves the use of doxycycline not for the treatment of an infection but as postexposure prophylactic (PEP) antibiotic therapy to prevent the acquisition of a sexually transmitted infection. Doxycycline PEP has been shown to reduce the rate of bacterial sexually transmitted infection in men who have sex with men (MSM). Doxycycline PEP is a single dose taken orally within 24–72 h of unprotected sexual intercourse. The dosing structure allows for ease of adherence but also repeatedly exposes individuals to doxycycline, putting them at risk for adverse events such as fixed drug eruptions, as illustrated by this case report.
Full article

Figure 1
Open AccessArticle
Assessment of the Utilization of Sodium–Glucose Cotransporter-2 Inhibitors in Patients Without Diabetes
by
Takuma Koinuma, Manato Yoshida and Manabu Akazawa
Pharmacoepidemiology 2024, 3(4), 383-393; https://doi.org/10.3390/pharma3040027 - 3 Dec 2024
Abstract
►▼
Show Figures
Background: Sodium–glucose cotransporter-2 inhibitors (SGLT2Is) have demonstrated effects beyond glucose-lowering, leading to their approval for treating chronic heart failure (HF) in Japan. This study examines prescription trends for SGLT2Is in patients with diabetes versus those without diabetes, focusing on their backgrounds and HF
[...] Read more.
Background: Sodium–glucose cotransporter-2 inhibitors (SGLT2Is) have demonstrated effects beyond glucose-lowering, leading to their approval for treating chronic heart failure (HF) in Japan. This study examines prescription trends for SGLT2Is in patients with diabetes versus those without diabetes, focusing on their backgrounds and HF treatment status of patients without diabetes who received SGLT2I after an HF diagnosis. Methods: Using data from DeSC Healthcare Inc., we analyzed patients aged 65 and above who received their first SGLT2I prescription between October 2014 and February 2023. Patients were classified into SGLT2I-treated diabetic and non-diabetic groups. We analyzed the annual prescription trends and compared the characteristics of both groups who started SGLT2I between 2022 and 2023. Additionally, we assessed the timing of SGLT2I initiation and the use of concomitant HF treatment in patients without diabetes after HF diagnosis. Results: The proportion of patients without diabetes receiving their first SGLT2I prescription has increased since 2021. Patients without diabetes receiving SGLT2Is were older, likely owing to aging-related diseases. In patients without a confirmed diabetes diagnosis, SGLT2I was most frequently initiated at the time of HF diagnosis. Mineralocorticoid receptor antagonists (MRAs) are the most common concomitant HF medications. The increase in SGLT2I prescriptions for patients without diabetes receiving SGLT2I since 2021, particularly in older individuals, suggests that SGLT2I is being initiated either at the time of HF diagnosis or in a stepwise manner. Conclusion: In Japan, MRA is commonly used as a concomitant medication in patients without diabetes receiving SGLT2I.
Full article

Figure 1
Open AccessArticle
Cardiovascular Adverse Events Associated with Prostate Cancer Treatment: A Disproportionality Analysis from the Food and Drug Administration Adverse Event Reporting System Database
by
Connor Frey
Pharmacoepidemiology 2024, 3(4), 373-382; https://doi.org/10.3390/pharma3040026 - 27 Nov 2024
Abstract
Background/Objectives: Several drugs used to treat prostate cancer have been reported to cause cardiovascular adverse events, and this study sought to identify the real-world risk. Methods: This study utilized real-world data from the FAERS to analyze the association between prostate cancer treatment and
[...] Read more.
Background/Objectives: Several drugs used to treat prostate cancer have been reported to cause cardiovascular adverse events, and this study sought to identify the real-world risk. Methods: This study utilized real-world data from the FAERS to analyze the association between prostate cancer treatment and cardiovascular adverse events. It evaluated men treated with LHRH agonists and antagonists, antiandrogens, androgen synthesis inhibitors, and PARP inhibitors from 2003 to 2023. This study included patients treated with leuprolide, goserelin, triptorelin, degarelix, relugolix, bicalutamide, flutamide, apalutamide, nilutamide, abiraterone, enzalutamide, olaparib, rucaparib, talazoparib, and niraparib. The main outcome measure was the reported odds ratio (ROR) of adverse cardiovascular event associated with these treatments. Results: Among the 4,049,329 unique adverse event reports, 4391 cardiovascular events were identified. Leuprolide (ROR 0.481, 95% CI: 0.423–0.547), triptorelin (ROR 0.527, 95% CI: 0.305–0.909), enzalutamide (ROR 0.393, 95% CI: 0.341–0.452), and olaparib (ROR 0.145, 95% CI: 0.054–0.386) reduced the risk of myocardial infarction. Goserelin increased the risk of myocardial infarction (ROR 2.235, 95% CI: 1.367–3.654). Degarelix and relugolix both increased the risk of heart failure (ROR 3.136, 95% CI: 2.186–4.497), and enzalutamide was associated with an increased risk of heart failure (ROR 1.305, 95% CI: 1.135–1.501). Bicalutamide increased the risk of unstable angina (ROR 3.019, 95% CI: 1.621–5.622) and heart failure (ROR 3.730, 95% CI: 3.085–4.510). Niraparib increased the risk of hypertension (ROR 4.154, 95% CI: 1.709–10.092). Conclusions: These findings underscore the need for clinicians to monitor cardiac complications in patients undergoing these therapies.
Full article
(This article belongs to the Topic Advance in Cancer Pharmacoepidemiology)
►▼
Show Figures

Graphical abstract
Open AccessOpinion
The Inappropriate Use of GLP-1 Analogs: Reflections from Pharmacoepidemiology
by
Sofía Echeverry-Guerrero, Salomé González-Vélez, Ana-Sofía Arévalo-Lara, Juan-Camilo Calvache-Orozco, Sebastián Kurt Villarroel-Hagemann, Luis Carlos Rojas-Rodríguez, Andrés M. Pérez-Acosta and Carlos-Alberto Calderon-Ospina
Pharmacoepidemiology 2024, 3(4), 365-372; https://doi.org/10.3390/pharma3040025 - 20 Nov 2024
Abstract
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have emerged as a potent therapeutic option for the management of obesity, demonstrating exceptional efficacy in several large-scale clinical trials. Despite their promising therapeutic outcomes, the rising popularity of these agents raises significant concerns, particularly regarding their
[...] Read more.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have emerged as a potent therapeutic option for the management of obesity, demonstrating exceptional efficacy in several large-scale clinical trials. Despite their promising therapeutic outcomes, the rising popularity of these agents raises significant concerns, particularly regarding their off-label use by individuals seeking weight loss for aesthetic reasons rather than addressing underlying metabolic health conditions. This article critically evaluates the efficacy and safety of GLP-1 RAs in obesity management. Additionally, it explores the economic implications and ethical challenges associated with the increasing demand for GLP-1 RAs. By addressing these dimensions, this article aims to facilitate informed and responsible decision-making in clinical practice, highlighting the need for individualized patient assessments and careful consideration of both short- and long-term safety risks.
Full article
Open AccessArticle
Can Pharmacovigilance Data Represent a Potential Tool for Early Detection of the Antibiotic Resistance Phenomenon?
by
Cecilia Cagnotta, Alessia Zinzi, Francesca Gargano, Valerio Liguori, Maria Rosaria Campitiello, Alessandro Perrella, Annalisa Capuano, Concetta Rafaniello and Ugo Trama
Pharmacoepidemiology 2024, 3(4), 350-364; https://doi.org/10.3390/pharma3040024 - 24 Oct 2024
Abstract
►▼
Show Figures
Background: Antibiotic resistance represents a growing concern. A new strategy developed to treat severe infections is represented by ceftazidime/avibactam (CZA/AVI). Despite the promising activities against more pathogens, continuous monitoring is required to identify potential antibiotic resistance in clinical practice settings. Therefore, real-world data
[...] Read more.
Background: Antibiotic resistance represents a growing concern. A new strategy developed to treat severe infections is represented by ceftazidime/avibactam (CZA/AVI). Despite the promising activities against more pathogens, continuous monitoring is required to identify potential antibiotic resistance in clinical practice settings. Therefore, real-world data from pharmacovigilance databases can help to better define the safety profile. Methods: We analyzed all Individual Case Safety Reports (ICSRs) collected in the EudraVigilance database focusing on ICSRs with at least one adverse event (AE) potentially suggestive of drug resistance (DR) and drug ineffectiveness (DI). Results: A total of 654 ICSRs related to CZA/AVI were retrieved from EudraVigilance, of which N = 378 (57.8%) were related to male and N = 230 (35.1%) to adult patients. A total of 80.2% of all AEs were serious but with a positive outcome. Overall, we found N = 129 (19.7%) cases of potential DR or DI after CZA/AVI administration. The majority of CZA/AVI-induced DR or DI occurred in adult male patients. The most frequently reported AEs were “drug ineffective” and “pathogen resistance”. Lastly, CZA/AVI was mostly used for the treatment of “Klebsiella infection” and “Pneumonia”. Conclusions: The present study showed how pharmacovigilance could play a key role in generating evidence about the safety profile of CZA/AVI. Further studies are warranted.
Full article

Figure 1
Open AccessReview
Maternal Medication Use in Pregnancy: A Narrative Review on Assessing and Communicating the “Risk” of Birth Defects to the Patient
by
Sura Alwan and Kimberly S. Grant
Pharmacoepidemiology 2024, 3(4), 336-349; https://doi.org/10.3390/pharma3040023 - 5 Oct 2024
Abstract
The state of knowledge regarding the teratogenic effects of maternal use of medications during pregnancy is constantly evolving and is often uncertain. Timely access to high-quality information may reduce prolonged harmful exposures, decrease the number of preventable birth defects, empower patients with accurate
[...] Read more.
The state of knowledge regarding the teratogenic effects of maternal use of medications during pregnancy is constantly evolving and is often uncertain. Timely access to high-quality information may reduce prolonged harmful exposures, decrease the number of preventable birth defects, empower patients with accurate information about the risks of exposure, and prevent unnecessary patient anxiety and pregnancy termination. In this narrative review, we describe the process by which the teratogenic risk of medications is assessed by experts in medicine, genetics, and epidemiology and how identifiable risks can be effectively communicated to patients. Risk assessment of birth defects in human pregnancy involves collecting and synthesizing available data through a proper and rule-driven evaluation of scientific literature. Expert consensus is a practical approach to determine whether a given exposure produces damage after careful consideration of gestational timing, dose and route of the exposure, maternal and fetal genetic susceptibility, as well as evidence for biological plausibility. The provision of teratogen risk counseling through appropriate interpretation of information and effective knowledge translation to the patient is critical for the prevention of birth defects and maximizing healthy pregnancies.
Full article
(This article belongs to the Special Issue Pharmacoepidemiology and Drug Safety in Pregnancy and Breastfeeding)
►▼
Show Figures

Figure 1
Open AccessArticle
Storage, Disposal, and Misuse of Unused and Expired Pharmaceuticals in Households amongst Staff Working at Dakshinapaya Ministry Complex, Labuduwa, Galle Region: A Qualitative Phenomenological Study
by
Pramila G. Chandrasena, Sampath Gunawardena and Shanika V. Karunanayaka
Pharmacoepidemiology 2024, 3(4), 314-335; https://doi.org/10.3390/pharma3040022 - 5 Oct 2024
Abstract
(1) Background: Although Sri Lanka is a developing country and boasts of having a well-established healthcare system along with good healthcare indices, we are still lagging in certain aspects of healthcare. One such aspect is the deficiencies in guidelines and practices related to
[...] Read more.
(1) Background: Although Sri Lanka is a developing country and boasts of having a well-established healthcare system along with good healthcare indices, we are still lagging in certain aspects of healthcare. One such aspect is the deficiencies in guidelines and practices related to the handling of pharmaceutical waste. (2) Methods: This was a qualitative study performed using in-depth interviews with the help of a semi-structured questionnaire conducted among staff who are working at a ministry complex in Galle, Sri Lanka. Data analysis was performed using thematic analysis, (3) Results: There were 40 participants which included 29 (72.5%) females. Three main themes were identified, namely, (I) current knowledge, (II) perceptions, and (III) practices towards storage, disposal, and misuse of pharmaceutical waste. The death of the patient, forgetting, relieving symptoms, and adverse effects were some reasons for the accumulation of unused pharmaceuticals at home. Most of the participants did not believe that the reuse of unused medications can cause various health hazards. Moreover, all participants practiced unsafe methods such as flushing down toilets, pouring into a sink, burning, etc. (4) Conclusions: The incorrect practices and poor knowledge in the handling of pharmaceutical waste and less concern for the environment highlight the need for awareness programs to the general public and establishing proper medication waste management such drug take-back systems.
Full article
Open AccessCase Report
Off-Label Use of Dalbavancin in Enterococcus spp. Abscess and Streptococcus pneumoniae Bacteremia Secondary to Septic Arthritis: A Retrospective Case Report
by
Miriam Banoub Morkos, Giovani Leon, Mai-Chi Hong, Joshua Allan Garcia, Martin J. Breen, Bhanu Sud and Lee Nguyen
Pharmacoepidemiology 2024, 3(4), 307-313; https://doi.org/10.3390/pharma3040021 - 29 Sep 2024
Abstract
Dalbavancin, a semi-synthetic lipoglycopeptide with an extended half-life that allows for weekly dosing, is currently approved for the treatment of bacterial skin and soft tissue infections caused by susceptible gram-positive organisms. This case report discusses the successful treatment of septic arthritis with dalbavancin
[...] Read more.
Dalbavancin, a semi-synthetic lipoglycopeptide with an extended half-life that allows for weekly dosing, is currently approved for the treatment of bacterial skin and soft tissue infections caused by susceptible gram-positive organisms. This case report discusses the successful treatment of septic arthritis with dalbavancin in a 38-year-old obese male. Septic arthritis, commonly caused by Staphylococcus aureus and Streptococcus species, was diagnosed in this patient following a mechanical fall that led to worsening shoulder pain. Given the patient’s morbid obesity and concerns about antibiotic penetration, dalbavancin 1500 mg IV biweekly was chosen for its extended half-life and ease of administration. This case underscores dalbavancin’s efficacy in managing septic arthritis in obese patients, offering a convenient alternative to traditional therapies that require a peripherally inserted central catheter (PICC line), frequent dosing, therapeutic monitoring, and prolonged hospital stays. Despite its higher cost, dalbavancin’s advantages include reduced need for PICC lines, additional staff and resources to monitor therapeutic drug levels, and fewer complications, which can offset some expenses. To our knowledge, this is the first documented case investigating the use of dalbavancin for enterococcal septic arthritis with a biweekly dosing regimen.
Full article
(This article belongs to the Special Issue Anti-Infectives: Pharmacoepidemiology and Clinical Pharmacology)
►▼
Show Figures

Figure 1
Open AccessArticle
Evaluating Public Behavior toward Antibiotic Use in Riyadh: A Cross-Sectional Study
by
Sarah A. Alfagih, Monirah A. Albabtain, Muaath Alfagih and Nouf Alharbi
Pharmacoepidemiology 2024, 3(3), 297-306; https://doi.org/10.3390/pharma3030020 - 13 Sep 2024
Abstract
►▼
Show Figures
Background: Antibiotic resistance presents a global challenge. Community awareness of antibiotic use has not been studied extensively in Saudi Arabia. This study aimed to assess public awareness of the appropriate use and indications of antibiotics in Riyadh, Saudi Arabia. Furthermore, the responses were
[...] Read more.
Background: Antibiotic resistance presents a global challenge. Community awareness of antibiotic use has not been studied extensively in Saudi Arabia. This study aimed to assess public awareness of the appropriate use and indications of antibiotics in Riyadh, Saudi Arabia. Furthermore, the responses were compared across gender and age groups. Methods: We conducted a cross-sectional study between September 2022 and October 2022, including adult participants from Riyadh. The questionnaires were distributed via electronic channels and included sections about participants’ sociodemographic data and behavior concerning antibiotic use. Results: This study included 453 respondents. There were 281 (62%) female and 172 (38%) male respondents. Most respondents were between 46 and 55 years (n = 111; 24.5%) and above 56 years (n = 134; 29.6%). Two hundred seventy-two (60%) were college/university graduates, and 113 (24.9%) were at the secondary school level. Most participants (n = 410; 90.5%) were not affiliated with or working in the health sector. One hundred thirty-nine (30.7%) participants used an antibiotic within the past six months, and 171 (37.7%) kept antibiotics at room temperature. Most participants (n = 380; 83.9%) completed the treatment plan as prescribed. Sixty-eight percent of respondents stopped taking antibiotics when they felt better, and 11.5% believed antibiotics can treat bacterial and viral infections. The responses were compared between patients of both genders and patients aged ≤55 years or older. The comparison indicated that females tended to store antibiotics as instructed by the manufacturer (p = 0.004) and disposed of the remaining antibiotics immediately after completing the treatment (p < 0.001). Furthermore, the indications for antibiotic use differed between the genders, with no difference between the age groups. Participants > 55 years tended to complete the treatment plan (p = 0.007) and continued taking antibiotics at the same time and dose as prescribed (p = 0.002). Conclusions: This study’s findings suggest that public health authorities should implement awareness intervention programs to educate the Riyadh community on the proper use of antibiotics, with target interventions for specific gender and age groups. This study’s findings should be interpreted in the context of the Riyadh community and the potential biases of cross-sectional studies.
Full article

Figure 1
Open AccessArticle
Benzodiazepine Adverse Reaction Cases Age 50 and Older Reported to the U.S. Poison Centers: Healthcare Use and Major Medical Effects
by
Bryan Y. Choi, Namkee G. Choi, C. Nathan Marti and S. David Baker
Pharmacoepidemiology 2024, 3(3), 285-296; https://doi.org/10.3390/pharma3030019 - 31 Aug 2024
Abstract
Background: Despite widespread consensus on the need to reduce benzodiazepine (BZD) use in older adults, prescription rates in the U.S. have paradoxically increased over the past few decades. Objective: We examined (1) the characteristics of the BZD adverse reaction cases in patients aged
[...] Read more.
Background: Despite widespread consensus on the need to reduce benzodiazepine (BZD) use in older adults, prescription rates in the U.S. have paradoxically increased over the past few decades. Objective: We examined (1) the characteristics of the BZD adverse reaction cases in patients aged 50 and older that were admitted to a healthcare facility (HCF) and experienced major effects/death, and (2) the associations between the concomitant use of opioids and/or antidepressants and HCF admission and major effects/death among BZD cases. Methods: We used the 2015–2022 National Poison Data System (NPDS), which contained data from 55 America’s Poison Centers. We fitted two multivariable logistic regression models to examine the associations between the co-use of opioids and/or antidepressants and HCF admission and major effects/death. Results: Of the BZD cases that were examined (N = 1979), 14.9% or 295 cases were admitted to healthcare facilities, and 8.5% of those who were followed up (77 out of 893 cases) experienced major effects or death. The number of co-used substances, co-use of opioids and antidepressants, atypical antipsychotics, anticonvulsants, muscle relaxants, and Gabapentin were associated with greater odds of healthcare admission. Co-use of opioids and healthcare admission were associated with greater odds of major effects/death. Conclusions: Adverse reactions and healthcare admissions are likely to be prevented when healthcare providers limit and carefully monitor BZD prescribing, especially for those who are on other medications, including prescription opioids and antidepressants.
Full article
Open AccessReview
Multi-Faceted Approach to Ventricular Tachycardia: A Review of Management Strategies
by
Francis Hartge, Jamario Skeete, Alejandro Pinedo, Bethlehem Zeleke, Asad Khan, Raktham Mekritthikrai and Cicely Anne Dye
Pharmacoepidemiology 2024, 3(3), 265-284; https://doi.org/10.3390/pharma3030018 - 12 Aug 2024
Abstract
►▼
Show Figures
Ventricular tachycardia poses a significant therapeutic challenge. It can manifest over a spectrum from minimal palpitation symptoms to sudden cardiac death. This makes large-scale trials on the treatment of ventricular tachycardia difficult to perform. The mechanism of ventricular tachycardia must also be understood
[...] Read more.
Ventricular tachycardia poses a significant therapeutic challenge. It can manifest over a spectrum from minimal palpitation symptoms to sudden cardiac death. This makes large-scale trials on the treatment of ventricular tachycardia difficult to perform. The mechanism of ventricular tachycardia must also be understood before embarking on treatment. Patients with or without structural heart disease will have different mechanisms for the onset and propagation of these arrhythmias. Catheter ablation is an established management option for ventricular tachycardia; however, it is not always successful and anti-arrhythmic medications are often necessary to control these life-threatening arrhythmias. Although anti-arrhythmics can suppress ventricular tachycardias they also carry side effects. In certain substrates, some of these medications can exacerbate arrhythmias or heart failure. For these reasons, a multifaceted approach to treating ventricular tachycardia is necessary. This paper is a comprehensive review of the comprehensive management strategies for ventricular tachycardia. Anti-arrhythmic medications have an important role and their use in various cardiomyopathies and channelopathies is reviewed in detail. We also review the promising effects of gene therapy and artificial intelligence on different substrates for ventricular tachycardia.
Full article

Figure 1
Open AccessReview
Utilization of Real-World Data to Facilitate Clinical Trials for Patients with Lymphoma
by
Dai Chihara, Brian P. Hobbs, Matthew J. Maurer and Christopher R. Flowers
Pharmacoepidemiology 2024, 3(3), 252-264; https://doi.org/10.3390/pharma3030017 - 6 Aug 2024
Abstract
The future directions in leveraging real-world evidence (RWE) and real-world data (RWD) in the field of lymphoma, as compared to traditional experimental clinical trials, are poised to significantly impact research methodologies, treatment strategies, and patient care. Current methods of clinical trials involve a
[...] Read more.
The future directions in leveraging real-world evidence (RWE) and real-world data (RWD) in the field of lymphoma, as compared to traditional experimental clinical trials, are poised to significantly impact research methodologies, treatment strategies, and patient care. Current methods of clinical trials involve a well-controlled design and patient selection bias. Integrating RWE and RWD with experimental clinical trials offers a multifaceted approach to understanding lymphoma and enhancing patient outcomes. In this review, we discuss how RWE has helped shape lymphoma clinical trials, and we compare and evaluate evidence obtained from real-world lymphoma studies/databases with that obtained from clinical trials. We also discuss methods for utilizing surrogate endpoints to facilitate clinical trials and expedite drug development. RWE can be leveraged to bridge the gap between data obtained from clinical trial populations and the broader patient population encountered in clinical practice, by highlighting differences in outcomes and the need for effective treatment strategies across diverse patient groups.
Full article
(This article belongs to the Special Issue Feature Papers of Pharmacoepidemiology)
Open AccessArticle
Associations between Suspected Adverse Drug Reactions of HMG-CoA Reductase Inhibitors and Polypharmacology Using a National Registry Approach
by
Hasan Yousaf and Alan M. Jones
Pharmacoepidemiology 2024, 3(3), 241-251; https://doi.org/10.3390/pharma3030016 - 3 Jul 2024
Abstract
Aims: The aim of this study was to explore the suspected adverse drug reaction (ADR) data of five licensed statins in the UK: atorvastatin, fluvastatin, pravastatin, rosuvastatin, and simvastatin. A secondary aim was to determine if there are any associations between the polypharmacological
[...] Read more.
Aims: The aim of this study was to explore the suspected adverse drug reaction (ADR) data of five licensed statins in the UK: atorvastatin, fluvastatin, pravastatin, rosuvastatin, and simvastatin. A secondary aim was to determine if there are any associations between the polypharmacological properties of the statins and their associated muscle-related side effects. Methods: The chemical database of bioactive molecules with drug-like properties, European Molecular Biology Laboratory (ChEMBL), was used to obtain data on the pharmacological interactions of statins with human proteins. The Medicines and Healthcare Products Regulatory Agency’s (MHRA) Yellow Card scheme was used to obtain reports of suspected ADRs from 2018 to 2022. The OpenPrescribing database was used to obtain the prescribing rates for statistical interpretation. Results: The study found no significant difference between the statins association with suspected ADRs across all organ classes (X2, p > 0.05). Fluvastatin was found to have a higher incidence of ADRs/100,000 Rx across multiple system organ classes. Conclusions: No significant difference was found between the suspected ADR incidence of the statins across all system organ classes.
Full article
Open AccessBrief Report
A Retrospective Analysis of the Clinical Effectiveness of Tigecycline in the Treatment of Clostridioides difficile-Associated Diarrhea
by
Herman Joseph Johannesmeyer, Luiza Baloyan and Kristica Kolyouthapong
Pharmacoepidemiology 2024, 3(2), 231-240; https://doi.org/10.3390/pharma3020015 - 13 Jun 2024
Abstract
Clostridioides difficile infection (CDI) is the leading cause of nosocomial diarrhea in the United States. Tigecycline has been proposed as a potential treatment for CDI, though limited clinical data exist to support this practice. The objective of this study was to determine if
[...] Read more.
Clostridioides difficile infection (CDI) is the leading cause of nosocomial diarrhea in the United States. Tigecycline has been proposed as a potential treatment for CDI, though limited clinical data exist to support this practice. The objective of this study was to determine if the provision of tigecycline provides a clinically meaningful benefit to inpatients with CDI. This study was a retrospective chart review enrolling inpatients receiving treatment for CDI. Patients were divided into cohorts depending on whether they received a standard antibiotic therapy regimen for CDI or an antibiotic treatment regimen that included tigecycline. The primary outcome was clinical recovery at the time of hospital discharge. A total of 39 and 22 patients were included in the standard antibiotic therapy and tigecycline groups, respectively. ATLAS (Age, Treatment, Leukocyte, Albumin, Serum creatinine) scores at the time of CDI diagnosis were similar between the two groups, though patients in the tigecycline groups were more likely to represent a recurrent episode of CDI. There was no difference in the rate of clinical recovery at the time of hospital discharge between the standard antibiotic therapy and tigecycline groups (38.5% vs. 36.4%, p = 0.8710). These data do not support the routine use of tigecycline for the treatment of CDI, though interpretation is limited due to baseline differences between groups and the retrospective, observational nature of this study.
Full article
(This article belongs to the Special Issue Anti-Infectives: Pharmacoepidemiology and Clinical Pharmacology)
►▼
Show Figures

Figure 1
Open AccessCommunication
Ocular Chloramphenicol Exposure in Early Childhood in Aotearoa/New Zealand
by
Isabella M. Y. Cheung, Simon Horsburgh, Ewan Smith, Samantha Simkin and Akilesh Gokul
Pharmacoepidemiology 2024, 3(2), 223-230; https://doi.org/10.3390/pharma3020014 - 14 May 2024
Abstract
►▼
Show Figures
Background: The paediatric use of ophthalmic chloramphenicol in New Zealand (NZ) is relatively high; however, little more is known about its utilisation, including whether this is equitable. This study aimed to describe chloramphenicol utilisation in NZ children aged five years and under, by
[...] Read more.
Background: The paediatric use of ophthalmic chloramphenicol in New Zealand (NZ) is relatively high; however, little more is known about its utilisation, including whether this is equitable. This study aimed to describe chloramphenicol utilisation in NZ children aged five years and under, by patient ethnicity, socioeconomic deprivation, and urban/non-urban domicile. Methods: This analysis included every publicly subsidised chloramphenicol dispensing received from birth to five years of age, for every child born in NZ in 2013. Cumulative proportion of first exposure, dispensing rate per person-year, and seasonality of dispensing were quantified. These were calculated following stratification by ethnicity, socioeconomic deprivation quintile, and urban/non-urban health district. For cumulative proportion of first exposure, odds ratios (OR) were calculated and multivariate logistic regression was performed. For dispensing rate, incidence rate ratios (IRR) were calculated and zero-inflated Poisson regression was performed. Results: Almost one-quarter of NZ children received their first dispensing within the first year of life. By five years of age, 55.2% of children had received their first dispensing. By five years of age, children of Pacific ethnicity, those in the highest deprivation quintile, and in those non-urban health districts had lower odds of receiving chloramphenicol (adjusted OR 0.90, 0.79, and 0.81, respectively, all p < 0.001). In contrast, children of Māori ethnicity had higher odds (adjusted OR 1.99, p < 0.001). Māori and Pacific ethnicity, and residence in non-urban health districts, were associated with fewer dispensings (adjusted IRR 0.88, 0.75 and 0.87, all p < 0.001). In contrast, deprivation quintile was not significantly associated with dispensing rate. Conclusion: Chloramphenicol utilisation is prevalent among NZ children, and utilisation may be lower among children of Pacific ethnicity and those in non-urban areas
Full article

Figure 1
Open AccessReview
Adverse Drug Reactions in Multimorbid Older People Exposed to Polypharmacy: Epidemiology and Prevention
by
Siobhán McGettigan, Denis Curtin and Denis O’Mahony
Pharmacoepidemiology 2024, 3(2), 208-222; https://doi.org/10.3390/pharma3020013 - 30 Apr 2024
Abstract
Adverse drug reactions (ADRs) are frequent and represent a significant healthcare burden. ADRs are a potentially avoidable contributor to excess unscheduled hospital admissions, higher morbidity, mortality, and healthcare costs. The objective of this review is to examine the epidemiology of ADRs in older
[...] Read more.
Adverse drug reactions (ADRs) are frequent and represent a significant healthcare burden. ADRs are a potentially avoidable contributor to excess unscheduled hospital admissions, higher morbidity, mortality, and healthcare costs. The objective of this review is to examine the epidemiology of ADRs in older multimorbid adults and to explore strategies for ADR prevention. ADRs in this population are often linked to commonly prescribed medications, including anticoagulants, antiplatelet agents, insulin, and non-steroidal anti-inflammatory drugs, but ADRs and adverse drug events (ADEs) in fact encompass a much broader range of culprit drugs. Age-related factors such as changes in pharmacokinetics and pharmacodynamics, multimorbidity, polypharmacy, and frailty have been associated with ADR occurrences. Various strategies have been proposed to prevent ADRs in different clinical settings, such as structured routine medication review and the use of bespoke software applications to identify potentially inappropriate prescriptions and drug interactions. Although these approaches have demonstrated some improvement in the quality of prescribing, there is still a lack of consistent evidence regarding their effectiveness in preventing ADRs. The nuanced and often intricate complexities associated with older patients’ pharmacotherapy necessitate a comprehensive approach to attenuate the impact of ADRs within this growing section of most populations globally.
Full article
(This article belongs to the Special Issue Feature Papers of Pharmacoepidemiology)
►▼
Show Figures
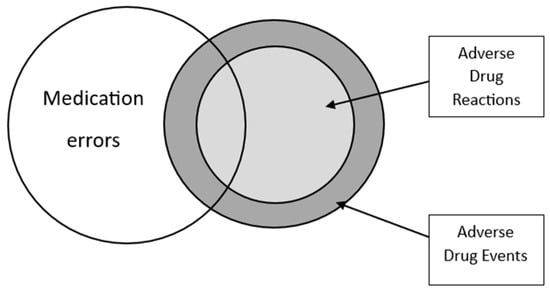
Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Cancers, Healthcare, Pharmaceuticals, Pharmaceutics, Pharmacoepidemiology
Advance in Cancer Pharmacoepidemiology
Topic Editors: Shih-Chieh Shao, Edward Chia-Cheng LaiDeadline: 20 February 2025

Conferences
Special Issues
Special Issue in
Pharmacoepidemiology
Pharmacoepidemiology and Pharmacovigilance in the UK
Guest Editor: Tanja MuellerDeadline: 28 February 2025
Special Issue in
Pharmacoepidemiology
Pharmacoepidemiology and Addiction—2nd Edition
Guest Editor: Matthew EllisDeadline: 31 May 2025
Special Issue in
Pharmacoepidemiology
Recent Advances in the Pharmacoepidemiology of Antirheumatic Medication
Guest Editors: Roberta Foti, Beatrice Maranini, Veronica VenturelliDeadline: 30 September 2025





