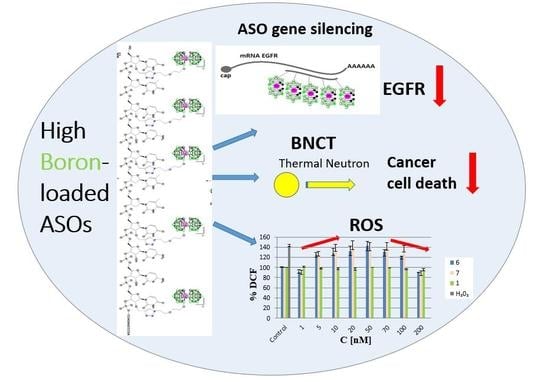
High Boron-loaded DNA-Oligomers as Potential Boron Neutron Capture Therapy and Antisense Oligonucleotide Dual-Action Anticancer Agents †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry: Synthesis of Boron Cluster Conjugated Oligonucleotides
2.2. Physicochemical Characterization of Oligomers 6 and 7
2.2.1. Lipophilicity of the Oligonucleotide Conjugates with Boron Clusters Measured as Affinity towards the Reversed Phase HPLC Column
2.2.2. Circular Dichroism (CD) Measurements of Boron Cluster-Conjugated Oligomers 6 and 7 with Their DNA and RNA Complementary Strands (2 and 3, Respectively)
2.2.3. Thermodynamic Properties of Duplexes of High Boron Cluster-Modified Antisense Oligomers 6 and 7 with DNA and RNA Templates
2.2.4. Infrared Spectroscopy Analysis
2.3. Biological Properties of High Boron Cluster-Loaded Antisense Oligonucleotides 6 and 7
2.3.1. Silencing Activity of Boron Cluster-Modified Anti-EGFR Antisense Oligonucleotides 6 and 7
2.3.2. Measurement of Reactive Oxygen Species (ROS) Generation by Metallacarborane, [(3,3′-Iron-1,2,1′,2′-dicarbollide)(−1)]ate Derivatives
2.3.3. Metabolic Activity of HeLa Cells Treated with Metallacarborane Derivatives
3. Materials and Methods
3.1 General Information
3.2. Mass Spectrometry and Ultraviolet Spectroscopy Measurements (UV)
3.3. Attenuated Total Reflectance−Fourier Transform Infrared (ATR−FTIR) Spectroscopy Measurements
3.4. Synthesis and Purification of DNA Oligonucleotides
3.5. Synthesis and Purification of DNA Oligonucleotides Modified with Boron Cluster
3.6. Electrophoretic Analysis Migration of ASOs in Polyacrylamide Gels
3.7. Melting Profiles and Thermodynamic Calculations
3.8. Chromatographic Analysis of Lipophilicity for Oligonucleotides 1 and 6–9
3.9. Circular Dichroism Measurements
3.10. Cell line and Cell Culture Conditions
3.11. Fluorescence Measurements of Silencing Activity of ASOs
3.12. Metabolic Activity of HeLa Cells Treated with Metallacarboranyl-Derivatives
3.13. Intracellular ROS Measurements in Living Cell
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mirzaei, H.R.; Sahebkar, A.; Salehi, R.; Nahand, J.S.; Karimi, E.; Jaafari, M.R.; Mirzaei, H. Boron neutron capture therapy: Moving toward targeted cancer therapy. J. Cancer Res. Ther. 2016, 12, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Coderre, J.A.; Vicente, M.G.H.; Blue, T.E. Boron neutron capture therapy of cancer: Current status and future prospects. Clin. Can. Res. Off. J. Am. Assoc. Can. Res. 2005, 11, 3987–4002. [Google Scholar] [CrossRef] [PubMed]
- Laurenţia, G.N.; Rodica, A.M. Boron neutron capture therapy: Delivery agents used in boron administration. Ther. Pharmacol. Clin. Toxicol. 2016, 20, 25–32. [Google Scholar]
- Sivaev, I.B.; Bregadze, V.V. Polyhedral boranes for medical applications: Current status and perspectives. Eur. J. Inorg. Chem. 2009, 2009, 1433–1450. [Google Scholar] [CrossRef]
- Harling, O.K.; Riley, K.J. Fission reactor neutron sources for neutron capture therapy—A critical review. J. Neuro-Oncol. 2003, 62, 7–17. [Google Scholar] [CrossRef]
- Adib, M.; Habib, N.; Bashter, I.I.; Mansy, M.S. Simulation study of accelerator based quasi-mono-energetic epithermal neutron beams for BNCT. Appl. Radiat. Isot. 2016, 107, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Taskaev, S.Y. Accelerator based epithermal neutron source. Phys. Part. Nucl. 2015, 46, 956–990. [Google Scholar] [CrossRef]
- Lesnikowski, Z.J.; Schinazi, R.F. Carboranyl Oligonucleotides. 1. Synthesis of Thymidine(3′,5′)thymidine (o-carboranyl-1-yl)methylphosphonate. J. Org. Chem. 1993, 58, 6531–6534. [Google Scholar] [CrossRef]
- Ebenryter-Olbińska, K.; Kaniowski, D.; Sobczak, M.; Wojtczak, B.; Janczak, S.; Wielgus, E.; Nawrot, B.; Leśnikowski, Z.J. Versatile method for the site specific modification of oligonucleotides with boron clusters—Anti-EGFR antisense oligonucleotide case. Chem. Eur. J. 2017. submitted. [Google Scholar]
- Baselga, J. Why the Epidermal Growth Factor Receptor? The Rationale for Cancer Therapy. Oncologist 2002, 2–8. [Google Scholar] [CrossRef]
- Okamoto, I. Epidermal growth factor receptor in relation to tumor development: EGFR-targeted anticancer therapy. FEBS J. 2010, 277, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Chen, Y.J.; Hung, M.C. Implication of nuclear EGFR in the development of resistance to anticancer therapies. BioMedicine 2011, 1, 2–10. [Google Scholar] [CrossRef]
- Zon, G.; Stec, W.J. Oligonucleotides and Analogues. A Practical Approach; Eckstein, F., Ed.; IRL Press at Oxford University Press: Oxford, UK, 1991; pp. 87–108. [Google Scholar]
- Meldal, M.; Tornoe, C.W. Cu-Catalyzed Azide-Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Wojtczak, B.A.; Olejniczak, A.B.; Lesnikowski, Z.J. Nucleoside modification with boron clusters and their metal complexes. In Current Protocols in Nucleic Acid Chemistry; Beaucage, S.L., Ed.; John Wiley & Sons, Inc.: Somerset, NJ, USA, 2009; Chapter 4, Unit 4.37; pp. 1–26. [Google Scholar]
- Amblard, F.; Cho, J.H.; Schinazi, R.F. The Cu (I)-catalyzed Huisgen azide-alkyne 1,3-dipolar cycloaddition reaction in nucleoside, nucleotide and oligonucleotide chemistry. Chem Rev. 2009, 109, 4207–4220. [Google Scholar] [CrossRef] [PubMed]
- Lesnikowski, Z.J. Boron clusters—A new entity for DNA-oligonucleotide modification. Eur. J. Org. Chem. 2003, 2003, 4489–4500. [Google Scholar] [CrossRef]
- Olejniczak, A.B.; Sut, A.; Wróblewski, A.E.; Leśnikowski, Z.J. Infrared spectroscopy of nucleoside and DNA-oligonucleotide conjugates labeled with carborane or metallacarborane group. Vib. Spectrosc. 2005, 39, 177–185. [Google Scholar] [CrossRef]
- Leites, L.A. Vibrational spectroscopy of carboranes and parent boranes and its capabilities in carborane chemistry. Chem. Rev. 1992, 92, 279–323. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Tournebize, J.; Sapin-Minet, A.; Bartosz, G.; Leroy, P.; Boudier, A. Pitfalls of assays devoted to evaluation of oxidative stress induced by inorganic nanoparticles. Talanta 2013, 116, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Morantes, C.Y.; Meléndez, E.; Singh, S.P.; Ramírez-Vick, J.E. Cytotoxicity and reactive oxygen species generated by ferrocenium and ferrocene on MCF7 and MCF10A cell lines. J. Cancer Sci. Ther. 2012, 4, 271. [Google Scholar] [CrossRef]
- Rinaldi, M.; Caffo, M.; Minutoli, L.; Marini, H.; Abbritti, R.V.; Squadrito, F.; Trichilo, V.; Valenti, A.; Barresi, V.; Altavilla, D.; et al. ROS and brain gliomas: An overview of potential and innovative therapeutic strategies. Int. J. Mol. Sci. 2016, 17, 984. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, M.; Zhang, B.; Zhang, Y. Reactive oxygen species regulate T cell immune response in the tumor microenvironment. Oxid. Med. Cell. Longev. 2016, 1580967. [Google Scholar] [CrossRef] [PubMed]
- Sierant, M.; Kubiak, K.; Kazmierczak-Baranska, J.; Warashina, M.; Kuwabara, T.; Nawrot, B. Evaluation of BACE1 Silencing in Cellular Models. Int. J. Alzheimers Dis. 2009, 2009. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–462. [Google Scholar] [CrossRef] [PubMed]
- Caruthers, M.H. Gene synthesis machines: DNA chemistry and its uses. Science 1985, 230, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Glen Research. Available online: http://www.glenresearch.com/GlenReports/GR25-24.html (accessed on 18 May 2017).
Sample Availability: Samples of the compound 10 are available from the authors. |













| No. | Sequence 3 | m/z Calc. | m/z MS MALDI | Rt 1 (min) | UV (nm) |
|---|---|---|---|---|---|
| 1 | 5′-d(TTT CTT TTC CTC CAG AGC CCGA)-3′ | 6612 | 6612 | 16.0 | 263 |
| 2 | 5′-d(AAA GAA AAG GAG GTC TCG GGCT)-3′ | 6857 | 6857 | 15.8 | 256 |
| 3 | 5′-AAA GAA AAG GAG GUC UCG GGCU-3′ | 7167 | 7168 | 15.3 | 256 |
| 4 | 5′-d(TUPrT CUPrT TUPrC CUPrC CAG AGC CCGA)-3′ | 6772 | 6775 | 16.4 | 263 |
| 5 | 5′-d(UPrTUPrCUPrTTUPrC CUPrC CAG AGC CCGA)-3′ | 6812 | 6813 | 16.5 | 264 |
| 6 | 5′-d(TUBT CUBT TUBC CUBC CAG AGC CCGA)-3′ | 8578 | 8575 | 22.2 | 263 |
| 7 | 5′-d(UBTUB CUBT TUBC CUBC CAG AGC CCGA)-3′ | 9068 | 9073 | 23.9 | 263 |
| 8 2 | 5′-d(TTT CTT UBTC CTC CAG AGC CCGA)-3′ | 6881 | 6884 | 17.5 | 263 |
| 9 2 | 5′-d(TTT CTT UBTC CUBC CAG AGC CCGA)-3′ | 7593 | 7598 | 20.1 | 263 |
| Duplex | ΔH [kcal/mol] | ΔS [cal/Kmol] | ΔG [kcal/mol] | Tm [°C] | ΔTm [°C] |
|---|---|---|---|---|---|
| 6/2 | −70.3 ± 4.0 | −192.0 ± 12.2 | −10.7 ± 0.2 | 49.5 ± 0.2 | −16.0 |
| 7/2 | −52.9 ± 1.0 | −136.1 ± 2.8 | −10.5 ± 0.1 | 51.9 ± 0.3 | −13.6 |
| 1/2 | −120.6 ± 8.6 | −330.1 ± 24.9 | −18.2 ± 0.9 | 65.5 ± 0.5 | − |
| 6/3 | −91.9 ± 1.3 | −249.3 ± 3.5 | −14.6 ± 0.2 | 60.9 ± 0.5 | −8.5 |
| 7/3 | −95.3 ± 7.0 | −259.8 ± 20.9 | −14.8 ± 0.5 | 60.4 ± 0.1 | −9.0 |
| 1/3 | −107.6 ± 6.8 | −288.2 ± 20.2 | −18.2 ± 0.6 | 69.4 ± 0.3 | − |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaniowski, D.; Ebenryter-Olbińska, K.; Sobczak, M.; Wojtczak, B.; Janczak, S.; Leśnikowski, Z.J.; Nawrot, B. High Boron-loaded DNA-Oligomers as Potential Boron Neutron Capture Therapy and Antisense Oligonucleotide Dual-Action Anticancer Agents. Molecules 2017, 22, 1393. https://doi.org/10.3390/molecules22091393
Kaniowski D, Ebenryter-Olbińska K, Sobczak M, Wojtczak B, Janczak S, Leśnikowski ZJ, Nawrot B. High Boron-loaded DNA-Oligomers as Potential Boron Neutron Capture Therapy and Antisense Oligonucleotide Dual-Action Anticancer Agents. Molecules. 2017; 22(9):1393. https://doi.org/10.3390/molecules22091393
Chicago/Turabian StyleKaniowski, Damian, Katarzyna Ebenryter-Olbińska, Milena Sobczak, Błażej Wojtczak, Sławomir Janczak, Zbigniew J. Leśnikowski, and Barbara Nawrot. 2017. "High Boron-loaded DNA-Oligomers as Potential Boron Neutron Capture Therapy and Antisense Oligonucleotide Dual-Action Anticancer Agents" Molecules 22, no. 9: 1393. https://doi.org/10.3390/molecules22091393
APA StyleKaniowski, D., Ebenryter-Olbińska, K., Sobczak, M., Wojtczak, B., Janczak, S., Leśnikowski, Z. J., & Nawrot, B. (2017). High Boron-loaded DNA-Oligomers as Potential Boron Neutron Capture Therapy and Antisense Oligonucleotide Dual-Action Anticancer Agents. Molecules, 22(9), 1393. https://doi.org/10.3390/molecules22091393