Fruit Seeds as Sources of Bioactive Compounds: Sustainable Production of High Value-Added Ingredients from By-Products within Circular Economy
Abstract
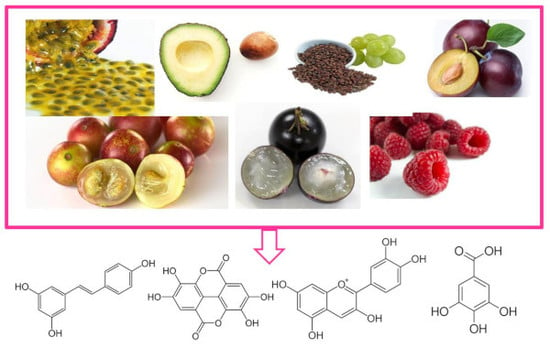
:1. Introduction
2. Fruit By-Products (Seeds): Chemical Characterization and Bioactivities
2.1. Apple (Pyrus malus L.)
2.2. Grape (Vitis Labrusca and Vitis Vinifera)
2.3. Pomegranate (Punica granatum)
2.4. Camu-Camu (Myrciaria Dubia H.B.K. McVaugh)
2.5. Plums (Prunus sp.)
2.6. Jabuticaba (Plinia jaboticaba and Plinia cauliflora Mart. Kausel)
2.7. Avocado (Persea americana Mill.)
2.8. Passion Fruit (Passiflora edulis Sims)
2.9. Berry Seeds
3. Extraction Technologies of Water-Soluble and Lipophilic Bioactive Compounds
4. Final Comments and Upcoming Research Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Homrich, A.S.; Galvão, G.; Abadia, L.G.; Carvalho, M.M. The circular economy umbrella: Trends and gaps on integrating pathways. J. Clean. Prod. 2018, 175, 525–543. [Google Scholar] [CrossRef]
- Moraga, G.; Huysveld, S.; Mathieux, F.; Blengini, G.A.; Alaerts, L.; Van Acker, K.; de Meester, S.; Dewulf, J. Circular economy indicators: What do they measure? Resour. Conserv. Recycl. 2019, 146, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.; McLaughlin, E.; Börger, T. The Circular Economy: Swings and Roundabouts? Ecol. Econ. 2019, 158, 11–19. [Google Scholar] [CrossRef]
- Pauliuk, S. Critical appraisal of the circular economy standard BS 8001:2017 and a dashboard of quantitative system indicators for its implementation in organizations. Resour. Conserv. Recycl. 2018, 129, 81–92. [Google Scholar] [CrossRef]
- Schroeder, P.; Anggraeni, K.; Weber, U. The Relevance of Circular Economy Practices to the Sustainable Development Goals. J. Ind. Ecol. 2019, 23, 77–95. [Google Scholar] [CrossRef]
- Save Food Infographics: Fruits and Vegetables Food Losses|SAVE FOOD: Global Initiative on Food Loss and Waste Reduction|Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/save-food/resources/keyfindings/infographics/fruit/en/ (accessed on 4 October 2019).
- About the Sustainable Development Goals - United Nations Sustainable Development. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 4 October 2019).
- Slorach, P.C.; Jeswani, H.K.; Cuéllar-Franca, R.; Azapagic, A. Environmental and economic implications of recovering resources from food waste in a circular economy. Sci. Total Environ. 2019, 693, 133516. [Google Scholar] [CrossRef]
- Velenturf, A.P.M.; Archer, S.A.; Gomes, H.I.; Christgen, B.; Lag-Brotons, A.J.; Purnell, P. Circular economy and the matter of integrated resources. Sci. Total Environ. 2019, 689, 963–969. [Google Scholar] [CrossRef]
- Rijswick, C. Rabobank Food and Agrobusiness. World Fruit Map. Rabobank. 2018. Available online: https://research.rabobank.com/far/en/sectors/regional-food-agri/world_fruit_map_2018.html (accessed on 4 October 2019).
- Bhushan, S.; Kalia, K.; Sharma, M.; Singh, B.; Ahuja, P.S. Processing of Apple Pomace for Bioactive Molecules. Crit. Rev. Biotechnol. 2008, 28, 285–296. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 4 October 2019).
- Deng, G.F.; Shen, C.; Xu, X.R.; Kuang, R.D.; Guo, Y.J.; Zeng, L.S.; Gao, L.L.; Lin, X.; Xie, J.F.; Xia, E.Q.; et al. Potential of fruit wastes as natural resources of bioactive compounds. Int. J. Mol. Sci. 2012, 13, 8308–8323. [Google Scholar] [CrossRef]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I.C.R.F. Bioactive characterization of Persea americana Mill. by-products: A rich source of inherent antioxidants. Ind. Crops Prod. 2018, 111, 212–218. [Google Scholar] [CrossRef]
- Fidelis, M.; Santos, J.S.; Escher, G.B.; Vieira do Carmo, M.; Azevedo, L.; Cristina da Silva, M.; Putnik, P.; Granato, D. In vitro antioxidant and antihypertensive compounds from camu-camu (Myrciaria dubia McVaugh, Myrtaceae) seed coat: A multivariate structure-activity study. Food Chem. Toxicol. 2018, 120, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Majerska, J.; Michalska, A.; Figiel, A. A review of new directions in managing fruit and vegetable processing by-products. Trends Food Sci. Technol. 2019, 88, 207–219. [Google Scholar] [CrossRef]
- Naeem, A.; Shabbir, M.A.; Khan, M.R.; Ahmad, N.; Roberts, T.H. Mango Seed Kernel Fat as a Cocoa Butter Substitute Suitable for the Tropics. J. Food Sci. 2019, 84, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [Green Version]
- Ayoub, M.; de Camargo, A.C.; Shahidi, F. Antioxidants and bioactivities of free, esterified and insoluble-bound phenolics from berry seed meals. Food Chem. 2016, 197, 221–232. [Google Scholar] [CrossRef]
- Karnopp, A.R.; Margraf, T.; Maciel, L.G.; Santos, J.S.; Granato, D. Chemical composition, nutritional and in vitro functional properties of by-products from the Brazilian organic grape juice industry. Int. Food Res. J. 2017, 24, 207–214. [Google Scholar]
- Tang, G.Y.; Zhao, C.N.; Liu, Q.; Feng, X.L.; Xu, X.Y.; Cao, S.Y.; Meng, X.; Li, S.; Gan, R.Y.; Li, H.B. Potential of grape wastes as a natural source of bioactive compounds. Molecules 2018, 23, 2598. [Google Scholar] [CrossRef]
- Karnopp, A.R.; Oliveira, K.G.; de Andrade, E.F.; Postingher, B.M.; Granato, D. Optimization of an organic yogurt based on sensorial, nutritional, and functional perspectives. Food Chem. 2017, 233, 401–411. [Google Scholar] [CrossRef]
- Karnopp, A.R.; Figueroa, A.M.; Los, P.R.; Teles, J.C.; Simões, D.R.S.; Barana, A.C.; Kubiaki, F.T.; de Oliveira, J.G.B.; Granato, D. Effects of whole-wheat flour and bordeaux grape pomace (Vitis labrusca L.) on the sensory, physicochemical and functional properties of cookies. Food Sci. Technol. 2015, 35, 750–756. [Google Scholar] [CrossRef]
- Sekhon-Loodu, S.; Warnakulasuriya, S.N.; Rupasinghe, H.P.V.; Shahidi, F. Antioxidant ability of fractionated apple peel phenolics to inhibit fish oil oxidation. Food Chem. 2013, 140, 189–196. [Google Scholar] [CrossRef]
- Santana, I.; Castelo-Branco, V.N.; Guimarães, B.M.; Silva, L.d.O.; Peixoto, V.O.D.S.; Cabral, L.M.C.; Freitas, S.P.; Torres, A.G. Hass avocado (Persea americana Mill.) oil enriched in phenolic compounds and tocopherols by expeller-pressing the unpeeled microwave dried fruit. Food Chem. 2019, 286, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Papadaki, A.; Bosnea, L.; Kanellaki, M.; Kopsahelis, N. Novel frozen yogurt production fortified with sea buckthorn berries and probiotics. LWT 2019, 105, 242–249. [Google Scholar] [CrossRef]
- Powell, M.J.; Sebranek, J.G.; Prusa, K.J.; Tarté, R. Evaluation of citrus fiber as a natural replacer of sodium phosphate in alternatively-cured all-pork Bologna sausage. Meat Sci. 2019, 157, 107883. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, L.; Innocenti, M.; Urciuoli, S.; Arlorio, M.; Paoli, P.; Mulinacci, N. In depth study of phenolic profile and PTP-1B inhibitory power of cold-pressed grape seed oils of different varieties. Food Chem. 2019, 271, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gu, B.-J.; Ganjyal, G.M. Impacts of the inclusion of various fruit pomace types on the expansion of corn starch extrudates. LWT 2019, 110, 223–230. [Google Scholar] [CrossRef]
- Ospina, M.; Montaña-Oviedo, K.; Díaz-Duque, Á.; Toloza-Daza, H.; Narváez-Cuenca, C.-E. Utilization of fruit pomace, overripe fruit, and bush pruning residues from Andes berry (Rubus glaucus Benth) as antioxidants in an oil in water emulsion. Food Chem. 2019, 281, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.; Jung, J.; Colonna, A.; Hasenbeck, A.; Gouw, V.; Zhao, Y. Quality and Consumer Acceptance of Berry Fruit Pomace-Fortified Specialty Mustard. J. Food Sci. 2018, 83, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Gouw, V.P.; Jung, J.; Zhao, Y. Functional properties, bioactive compounds, and in vitro gastrointestinal digestion study of dried fruit pomace powders as functional food ingredients. LWT 2017, 80, 136–144. [Google Scholar] [CrossRef]
- Rtibi, K.; Selmi, S.; Saidani, K.; Grami, D.; Amri, M.; Sebai, H.; Marzouki, L. Reverse Effect of Opuntia ficus-indica L. Juice and Seeds Aqueous Extract on Gastric Emptying and Small-Bowel Motility in Rat. J. Food Sci. 2018, 83, 205–211. [Google Scholar] [CrossRef]
- Marques, T.R.; Cesar, P.H.S.; Braga, M.A.; Marcussi, S.; Corrêa, A.D. Fruit Bagasse Phytochemicals from Malpighia Emarginata Rich in Enzymatic Inhibitor with Modulatory Action on Hemostatic Processes. J. Food Sci. 2018, 83, 2840–2849. [Google Scholar] [CrossRef]
- View of Phenolic Content, Antioxidant and Anti-Inflammatory Activities of Seeds and Leaves of Date Palm (Phoenix dactylifera L.). Available online: http://www.isnff-jfb.com/index.php/JFB/article/view/72/137 (accessed on 3 October 2019).
- Gong, X.; Tang, M.; Gong, Z.; Qiu, Z.; Wang, D.; Fan, M. Screening pesticide residues on fruit peels using portable Raman spectrometer combined with adhesive tape sampling. Food Chem. 2019, 295, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Sirisena, S.; Zabaras, D.; Ng, K.; Ajlouni, S. Characterization of Date (Deglet Nour) Seed Free and Bound Polyphenols by High-Performance Liquid Chromatography-Mass Spectrometry. J. Food Sci. 2017, 82, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Takii, H.; Matsumoto, K.; Kometani, T.; Okada, S.; Fushiki, T. Lowering Effect of Phenolic Glycosides on the Rise in Postprandial Glucose in Mice. Biosci. Biotechnol. Biochem. 1997, 61, 1531–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, Y.S.; Sia, C.M.; Khoo, H.E.; Ang, Y.K.; Chang, S.K.; Chang, S.K.; Yim, H.S. Influence of extraction conditions on antioxidant properties of passion fruit (Passiflora edulis) peel. Acta Sci. Pol. Technol. Aliment. 2014, 13, 257–265. [Google Scholar] [CrossRef]
- Dorta, E.; Lobo, M.G.; Gonzalez, M. Reutilization of Mango Byproducts: Study of the Effect of Extraction Solvent and Temperature on Their Antioxidant Properties. J. Food Sci. 2012, 77, C80–C88. [Google Scholar] [CrossRef]
- Durante, M.; Montefusco, A.; Marrese, P.P.; Soccio, M.; Pastore, D.; Piro, G.; Mita, G.; Lenucci, M.S. Seeds of pomegranate, tomato and grapes: An underestimated source of natural bioactive molecules and antioxidants from agri-food by-products. J. Food Compos. Anal. 2017, 63, 65–72. [Google Scholar] [CrossRef]
- Derakhshan, Z.; Ferrante, M.; Tadi, M.; Ansari, F.; Heydari, A.; Hosseini, M.S.; Conti, G.O.; Sadrabad, E.K. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem. Toxicol. 2018, 114, 108–111. [Google Scholar] [CrossRef]
- Van Nieuwenhove, C.P.; Moyano, A.; Castro-Gómez, P.; Fontecha, J.; Sáez, G.; Zárate, G.; Pizarro, P.L. Comparative study of pomegranate and jacaranda seeds as functional components for the conjugated linolenic acid enrichment of yogurt. LWT 2019, 111, 401–407. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Identification of phenolic antioxidants and bioactives of pomegranate seeds following juice extraction using HPLC-DAD-ESI-MS n. Food Chem. 2017, 221, 1883–1894. [Google Scholar] [CrossRef]
- Yoshime, L.T.; de Melo, I.L.P.; Sattler, J.A.G.; Torres, R.P.; Mancini-Filho, J. Bioactive compounds and the antioxidant capacities of seed oils from pomegranate (Punica granatum L.) and bitter gourd (Momordica charantia L.). Food Sci. Technol. 2018. Ahead of Print. [Google Scholar] [CrossRef]
- Myoda, T.; Fujimura, S.; Park, B.J.; Nagashima, T.; Nakagawa, J.; Nishizawa, M. Antioxidative and antimicrobial potential of residues of camu-camu juice production. J. Food, Agric. Environ. 2010, 8, 304–307. [Google Scholar]
- Azevêdo, J.C.S.; Fujita, A.; de Oliveira, E.L.; Genovese, M.I.; Correia, R.T.P. Dried camu-camu (Myrciaria dubia H.B.K. McVaugh) industrial residue: A bioactive-rich Amazonian powder with functional attributes. Food Res. Int. 2014, 62, 934–940. [Google Scholar] [CrossRef]
- Maria, L.G.; Edvan, A.C.; Bala, R.; Pollyana, C.C.; Antonia, R.V.d.S.; Sara, T.M.S.; Railin, R.d.O. Qualitative evaluation and biocompounds present in different parts of camu-camu (Myrciaria dubia) fruit. Afr. J. Food Sci. 2017, 11, 124–129. [Google Scholar] [CrossRef]
- Do Carmo, M.A.V.D.; Fidelis, M.; Pressete, C.G.; Marques, M.J.; Castro-Gamero, A.M.; Myoda, T.; Granato, D.; Azevedo, L. Hydroalcoholic Myrciaria dubia (camu-camu) seed extracts prevent chromosome damage and act as antioxidant and cytotoxic agents. Food Res. Int. 2019, 125, 108551. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, M.; Ran, J.; Zhang, T.; Sun, H.; Dong, M.; Zhang, Z.; Zheng, H. Variation in phenolic compounds and antioxidant activity in apple seeds of seven cultivars. Saudi J. Biol. Sci. 2016, 23, 379–388. [Google Scholar] [CrossRef]
- Alkhalf, M.I.; Alansari, W.S.; Ibrahim, E.A.; ELhalwagy, M.E.A. Anti-oxidant, anti-inflammatory and anti-cancer activities of avocado (Persea americana) fruit and seed extract. J. King Saud Univ. - Sci. 2018. [Google Scholar] [CrossRef]
- Fromm, M.; Bayha, S.; Carle, R.; Kammerer, D.R. Characterization and Quantitation of Low and High Molecular Weight Phenolic Compounds in Apple Seeds. J. Agric. Food Chem. 2012, 60, 1232–1242. [Google Scholar] [CrossRef]
- Paludo, M.; Colombo, R.; Teixeira, J.; Hermosín-Gutiérrez, I.; Ballus, C.; Godoy, H.; Paludo, M.C.; Colombo, R.C.; Teixeira Filho, J.; Hermosín-Gutiérrez, I.; et al. Optimizing the Extraction of Anthocyanins from the Skin and Phenolic Compounds from the Seed of Jabuticaba Fruits (Myrciaria jabuticaba (Vell.) O. Berg) with Ternary Mixture Experimental Designs. J. Braz. Chem. Soc. 2019, 30, 1506–1515. [Google Scholar] [CrossRef]
- Inada, K.O.P.; Oliveira, A.A.; Revorêdo, T.B.; Martins, A.B.N.; Lacerda, E.C.Q.; Freire, A.S.; Braz, B.F.; Santelli, R.E.; Torres, A.G.; Perrone, D.; et al. Screening of the chemical composition and occurring antioxidants in jabuticaba (Myrciaria jaboticaba) and jussara (Euterpe edulis) fruits and their fractions. J. Funct. Foods 2015, 17, 422–433. [Google Scholar] [CrossRef]
- Hacke, A.C.M.; Granato, D.; Maciel, L.G.; Weinert, P.L.; do Prado-Silva, L.; Alvarenga, V.O.; de Souza Sant’Ana, A.; Bataglion, G.A.; Eberlin, M.N.; Rosso, N.D. Jabuticaba (Myrciaria cauliflora) Seeds: Chemical Characterization and Extraction of Antioxidant and Antimicrobial Compounds. J. Food Sci. 2016, 81, C2206–C2217. [Google Scholar] [CrossRef]
- Alezandro, M.R.; Dubé, P.; Desjardins, Y.; Lajolo, F.M.; Genovese, M.I. Comparative study of chemical and phenolic compositions of two species of jaboticaba: Myrciaria jaboticaba (Vell.) Berg and Myrciaria cauliflora (Mart.) O. Berg. Food Res. Int. 2013, 54, 468–477. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Kean, C. Polyphenol concentrations in apple processing by-products determined using electrospray ionization mass spectrometry. Can. J. Plant Sci. 2008, 88, 759–762. [Google Scholar] [CrossRef]
- Jham, G.N. High-performance liquid chromatographic quantitation of phloridzin in apple seed, leaf and callus. J. Chromatogr. A 1996, 719, 444–449. [Google Scholar] [CrossRef]
- Rosero, J.C.; Cruz, S.; Osorio, C.; Hurtado, N. Analysis of Phenolic Composition of Byproducts (Seeds and Peels) of Avocado (Persea americana Mill.) Cultivated in Colombia. Molecules 2019, 24, 3209. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Comprehensive characterization of phenolic and other polar compounds in the seed and seed coat of avocado by HPLC-DAD-ESI-QTOF-MS. Food Res. Int. 2018, 105, 752–763. [Google Scholar] [CrossRef]
- Athaydes, B.R.; Alves, G.M.; de Assis, A.L.E.M.; Gomes, J.V.D.; Rodrigues, R.P.; Campagnaro, B.P.; Nogueira, B.V.; Silveira, D.; Kuster, R.M.; Pereira, T.M.C.; et al. Avocado seeds (Persea americana Mill.) prevents indomethacin-induced gastric ulcer in mice. Food Res. Int. 2019, 119, 751–760. [Google Scholar] [CrossRef]
- Segovia, F.J.; Corral-Pérez, J.J.; Almajano, M.P. Avocado seed: Modeling extraction of bioactive compounds. Ind. Crops Prod. 2016. [Google Scholar] [CrossRef]
- Amariz, A.; de Lima, M.A.C.; Alves, R.E. Quality and antioxidant potential of byproducts from refining of fruit pulp. Food Sci. Technol. 2018, 38, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Deolindo, C.T. P.; Monteiro, P.I.; Santos, J.S.; Cruz, A.G.; Cristina da Silva, M.; Granato, D. Phenolic-rich Petit Suisse cheese manufactured with organic Bordeaux grape juice, skin, and seed extract: Technological, sensory, and functional properties. LWT 2019, 115, 108493. [Google Scholar] [CrossRef]
- Harbeoui, H.; Hichami, A.; Wannes, W.A.; Lemput, J.; Tounsi, M.S.; Khan, N.A. Anti-inflammatory effect of grape (Vitis vinifera L.) seed extract through the downregulation of NF-κB and MAPK pathways in LPS-induced RAW264.7 macrophages. S. Afr. J. Bot. 2019, 125, 1–8. [Google Scholar] [CrossRef]
- De Albuquerque, M.A.C.; Levit, R.; Beres, C.; Bedani, R.; de Moreno de LeBlanc, A.; Saad, S.M.I.; LeBlanc, J.G. Tropical fruit by-products water extracts as sources of soluble fibres and phenolic compounds with potential antioxidant, anti-inflammatory, and functional properties. J. Funct. Foods 2019, 52, 724–733. [Google Scholar] [CrossRef]
- Saravanan, S.; Parimelazhagan, T. Total phenolic content, Free radical scavenging and Antimicrobial activities of Passiflora subpeltata seeds. J. Appl. Pharm. Sci. 2013, 3, 67–72. [Google Scholar]
- Meini, M.-R.; Cabezudo, I.; Boschetti, C.E.; Romanini, D. Recovery of phenolic antioxidants from Syrah grape pomace through the optimization of an enzymatic extraction process. Food Chem. 2019, 283, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.A.; Angonese, M.; Gomes, C.; Ferreira, S.R.S. Valorization of passion fruit (Passiflora edulis sp.) by-products: Sustainable recovery and biological activities. J. Supercrit. Fluids 2016, 111, 55–62. [Google Scholar] [CrossRef]
- Rotta, E.M.; Rodrigues, C.A.; Jardim, I.C.S.F.; Maldaner, L.; Visentainer, J.V. Determination of phenolic compounds and antioxidant activity in passion fruit pulp (Passiflora spp.) using a modified QuEChERS method and UHPLC-MS/MS. LWT 2019, 100, 397–403. [Google Scholar] [CrossRef]
- Schieber, A.; Hilt, P.; Streker, P.; Endreß, H.-U.; Rentschler, C.; Carle, R. A new process for the combined recovery of pectin and phenolic compounds from apple pomace. Innov. Food Sci. Emerg. Technol. 2003, 4, 99–107. [Google Scholar] [CrossRef]
- Lu, Y.; Yeap Foo, L. Constitution of some chemical components of apple seed. Food Chem. 1998, 61, 29–33. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzińska, M.; Soliven, A. Industrial by-products of plum Prunus domestica L. and Prunus cerasifera Ehrh. as potential biodiesel feedstock: Impact of variety. Ind. Crops Prod. 2017, 100, 77–84. [Google Scholar] [CrossRef]
- Skinner, R.; Warren, D.; Lateef, S.; Benedito, V.; Tou, J. Apple Pomace Consumption Favorably Alters Hepatic Lipid Metabolism in Young Female Sprague-Dawley Rats Fed a Western Diet. Nutrients 2018, 10, 1882. [Google Scholar] [CrossRef]
- Walia, M.; Rawat, K.; Bhushan, S.; Padwad, Y.S.; Singh, B. Fatty acid composition, physicochemical properties, antioxidant and cytotoxic activity of apple seed oil obtained from apple pomace. J. Sci. Food Agric. 2014, 94, 929–934. [Google Scholar] [CrossRef]
- Bada, J.C.; León-Camacho, M.; Copovi, P.; Alonso, L. Characterization of apple seed oil with Denomination of Origin from Asturias, Spain. Grasas y Aceites 2014, 65, e027. [Google Scholar] [Green Version]
- Matthäus, B.; Musazcan Özcan, M. Oil Content, Fatty Acid Composition and Distributions of Vitamin-E-Active Compounds of Some Fruit Seed Oils. Antioxidants 2015, 4, 124–133. [Google Scholar]
- Tian, H.-L.; Zhan, P.; Li, K.-X. Analysis of components and study on antioxidant and antimicrobial activities of oil in apple seeds. Int. J. Food Sci. Nutr. 2010, 61, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; van de Voort, F.R.; Li, Z.; Yue, T. Proximate Composition of the Apple Seed and Characterization of Its Oil. Int. J. Food Eng. 2007, 3. [Google Scholar] [CrossRef]
- Górnaś, P. Unique variability of tocopherol composition in various seed oils recovered from by-products of apple industry: Rapid and simple determination of all four homologues (α, β, γ and δ) by RP-HPLC/FLD. Food Chem. 2015, 172, 129–134. [Google Scholar] [CrossRef]
- Opyd, P.; Jurgoński, A.; Juśkiewicz, J.; Milala, J.; Zduńczyk, Z.; Król, B. Nutritional and Health-Related Effects of a Diet Containing Apple Seed Meal in Rats: The Case of Amygdalin. Nutrients 2017, 9, 1091. [Google Scholar] [CrossRef]
- Gosch, C.; Halbwirth, H.; Stich, K. Phloridzin: Biosynthesis, distribution and physiological relevance in plants. Phytochemistry 2010, 71, 838–843. [Google Scholar] [CrossRef]
- Ehrenkranz, J.R.L.; Lewis, N.G.; Ronald Kahn, C.; Roth, J. Phlorizin: A review. Diabetes. Metab. Res. Rev. 2005, 21, 31–38. [Google Scholar] [CrossRef]
- Rossetti, L.; Smith, D.; Shulman, G.I.; Papachristou, D.; DeFronzo, R.A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J. Clin. Investig. 1987, 79, 1510–1515. [Google Scholar] [CrossRef]
- Rossetti, L.; Shulman, G.I.; Zawalich, W.; DeFronzo, R.A. Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J. Clin. Investig. 1987, 80, 1037–1044. [Google Scholar] [CrossRef]
- Shin, S.-K.; Cho, S.-J.; Jung, U.; Ryu, R.; Choi, M.-S. Phlorizin Supplementation Attenuates Obesity, Inflammation, and Hyperglycemia in Diet-Induced Obese Mice Fed a High-Fat Diet. Nutrients 2016, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R.A. Amygdalin content of seeds, kernels and food products commercially-available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speijers, G. Cyanogenic glycosides. WHO JECFA Food Add. Ser. 1993, 30, 299–337. Available online: http://www.inchem.org/documents/jecfa/jecmono/v30je18.htm (accessed on 3 October 2019).
- Zhou, K.; Raffoul, J.J. Potential Anticancer Properties of Grape Antioxidants. J. Oncol. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, J.; Chen, Q.; Tao, T.; Wang, G.; Xu, F. Promotive effects of 5-Aminolevulinic acid on growth, photosynthetic gas exchange, chlorophyll, and antioxidative enzymes under salinity stress in Prunnus persica (L.) Batseh Seedling. Emirates J. Food Agric. 2016, 28, 786–795. [Google Scholar] [CrossRef]
- The Plant List. 2013. Available online: http://www.theplantlist.org/ (accessed on 3 October 2019).
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D. V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Vitis vinifera (Grape)-Derived Ingredients as Used in Cosmetics. Int. J. Toxicol. 2014, 33, 48S–83S. [Google Scholar] [CrossRef] [PubMed]
- Asensi, M.; Medina, I.; Ortega, A.; Carretero, J.; Baño, M.C.; Obrador, E.; Estrela, J.M. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic. Biol. Med. 2002, 33, 387–398. [Google Scholar] [CrossRef]
- Hakimuddin, F.; Tiwari, K.; Paliyath, G.; Meckling, K. Grape and wine polyphenols down-regulate the expression of signal transduction genes and inhibit the growth of estrogen receptor–negative MDA-MB231 tumors in nu/nu mouse xenografts. Nutr. Res. 2008, 28, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Velmurugan, B.; Rajamanickam, S.; Agarwal, R.; Agarwal, C. Gallic Acid, an Active Constituent of Grape Seed Extract, Exhibits Anti-proliferative, Pro-apoptotic and Anti-tumorigenic Effects Against Prostate Carcinoma Xenograft Growth in Nude Mice. Pharm. Res. 2009, 26, 2133–2140. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Hall, P.; Smith, M.; Kirk, M.; Prasain, J.K.; Barnes, S.; Grubbs, C. Chemoprevention by Grape Seed Extract and Genistein in Carcinogen-induced Mammary Cancer in Rats Is Diet Dependent. J. Nutr. 2004, 134, 3445S–3452S. [Google Scholar] [CrossRef]
- Morré, D.M.; Morré, D.J. Anticancer activity of grape and grape skin extracts alone and combined with green tea infusions. Cancer Lett. 2006, 238, 202–209. [Google Scholar] [CrossRef]
- Singh, T.; Sharma, S.D.; Katiyar, S.K. Grape Proanthocyanidins Induce Apoptosis by Loss of Mitochondrial Membrane Potential of Human Non-Small Cell Lung Cancer Cells In vitro and In vivo. PLoS ONE 2011, 6, e27444. [Google Scholar] [CrossRef] [PubMed]
- Schlachterman, A.; Valle, F.; Wall, K.M.; Azios, N.G.; Castillo, L.; Morell, L.; Washington, A.V.; Cubano, L.A.; Dharmawardhane, S.F. Combined Resveratrol, Quercetin, and Catechin Treatment Reduces Breast Tumor Growth in a Nude Mouse Model. Transl. Oncol. 2008, 1, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singletary, K.W.; Stansbury, M.J.; Giusti, M.; van Breemen, R.B.; Wallig, M.; Rimando, A. Inhibition of Rat Mammary Tumorigenesis by Concord Grape Juice Constituents. J. Agric. Food Chem. 2003, 51, 7280–7286. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Karlsson, R.M.; Oksman, P.H.; Kallio, H.P. Phytosterols in Sea Buckthorn (Hippophaë rhamnoides L.) Berries: Identification and Effects of Different Origins and Harvesting Times. J. Agric. Food Chem. 2001, 49, 5620–5629. [Google Scholar] [CrossRef]
- Burin, V.M.; Ferreira-Lima, N.E.; Panceri, C.P.; Bordignon-Luiz, M.T. Bioactive compounds and antioxidant activity of Vitis vinifera and Vitis labrusca grapes: Evaluation of different extraction methods. Microchem. J. 2014, 114, 155–163. [Google Scholar] [CrossRef]
- Granato, D.; Koot, A.; Schnitzler, E.; van Ruth, S.M. Authentication of geographical origin and crop system of grape juices by phenolic compounds and antioxidant activity using chemometrics. J. Food Sci. 2015, 80, C584–C593. [Google Scholar] [CrossRef]
- Salehi, B.; Vlaisavljevic, S.; Adetunji, C.O.; Adetunji, J.B.; Kregiel, D.; Antolak, H.; Pawlikowska, E.; Uprety, Y.; Mileski, K.S.; Devkota, H.P.; et al. Plants of the genus Vitis: Phenolic compounds, anticancer properties and clinical relevance. Trends Food Sci. Technol. 2019, 91, 362–379. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Nowicka, P.; Turkiewicz, I.; Golis, T. Characterization in vitro potency of biological active fractions of seeds, skins and flesh from selected Vitis vinifera L. cultivars and interspecific hybrids. J. Funct. Foods 2019, 56, 353–363. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Göksel, Z.; Erdoğan, S.S.; Öztürk, A.; Atak, A.; Özer, C. Antioxidant Activity and Phenolic Content of Seed, Skin and Pulp Parts of 22 Grape (Vitis vinifera L.) Cultivars (4 Common and 18 Registered or Candidate for Registration). J. Food Process. Preserv. 2015, 39, 1682–1691. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape Pomace as a Sustainable Source of Bioactive Compounds: Extraction, Characterization, and Biotechnological Applications of Phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of polyphenol-rich grape by-products in monogastric nutrition. A review. Anim. Feed Sci. Technol. 2016, 211, 1–17. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M.; Coïsson, J.D.; Arlorio, M. Characterisation of polymeric skin and seed proanthocyanidins during ripening in six Vitis vinifera L. cv. Food Chem. 2011, 127, 180–187. [Google Scholar] [CrossRef]
- Qiang, X.; YongLie, C.; QianBing, W. Health benefit application of functional oligosaccharides. Carbohydr. Polym. 2009, 77, 435–441. [Google Scholar] [CrossRef]
- Bordiga, M. Valorization of Wine Making By-Products; CRC Press, Taylor & Francis Group: Novara, Italy, 2015. [Google Scholar]
- Bordiga, M.; Meudec, E.; Williams, P.; Montella, R.; Travaglia, F.; Arlorio, M.; Coïsson, J.D.; Doco, T. The impact of distillation process on the chemical composition and potential prebiotic activity of different oligosaccharidic fractions extracted from grape seeds. Food Chem. 2019, 285, 423–430. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Olive by-products for functional and food applications: Challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Andrade, M.A.; Lima, V.; Sanches Silva, A.; Vilarinho, F.; Castilho, M.C.; Khwaldia, K.; Ramos, F. Pomegranate and grape by-products and their active compounds: Are they a valuable source for food applications? Trends Food Sci. Technol. 2019, 86, 68–84. [Google Scholar] [CrossRef]
- Aziz, A.R.A.; AbouLaila, M.R.; Aziz, M.; Omar, M.A.; Sultan, K. In vitro and in vivo anthelmintic activity of pumpkin seeds and pomegranate peels extracts against Ascaridia galli. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 231–234. [Google Scholar] [CrossRef]
- Białek, A.; Stawarska, A.; Bodecka, J.; Białek, M.; Tokarz, A. Pomegranate seed oil influences the fatty acids profile and reduces the activity of desaturases in livers of Sprague-Dawley rats. Prostaglandins Other Lipid Mediat. 2017, 131, 9–16. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; van Camp, J.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Sari, M.H.M.; Cervi, V.F.; Gehrcke, M.; Barbieri, A.V.; Zborowski, V.A.; Beck, R.C.R.; Nogueira, C.W.; Cruz, L. Pomegranate seed oil nanoemulsions improve the photostability and in vivo antinociceptive effect of a non-steroidal anti-inflammatory drug. Colloids Surf. B Biointerfaces 2016, 144, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Zarepourfard, H.; Riasi, A.; Frouzanfar, M.; Hajian, M.; Nasr Esfahani, M.H. Pomegranate seed in diet, affects sperm parameters of cloned goats following freezing-thawing. Theriogenology 2019, 125, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.S.; Oh, S.; Eun, J.B.; Ahmed, M. Nutritional compositions and health promoting phytochemicals of camu-camu (Myrciaria dubia) fruit: A review. Food Res. Int. 2011, 44, 1728–1732. [Google Scholar] [CrossRef]
- Kaneshima, T.; Myoda, T.; Nakata, M.; Fujimori, T.; Toeda, K.; Nishizawa, M. Antioxidant activity of C-Glycosidic ellagitannins from the seeds and peel of camu-camu (Myrciaria dubia). LWT - Food Sci. Technol. 2016, 69, 76–81. [Google Scholar] [CrossRef]
- Camere-Colarossi, R.; Ulloa-Urizar, G.; Medina-Flores, D.; Caballero-García, S.; Mayta-Tovalino, F.; del Valle-Mendoza, J. Antibacterial activity of Myrciaria dubia (Camu camu) against Streptococcus mutans and Streptococcus sanguinis. Asian Pac. J. Trop. Biomed. 2016, 6, 740–744. [Google Scholar] [CrossRef]
- Yazawa, K.; Suga, K.; Honma, A.; Shirosaki, M.; Koyama, T. Anti-inflammatory effects of seeds of the tropical fruit camu-camu (Myrciaria dubia). J. Nutr. Sci. Vitaminol. (Tokyo) 2011, 57, 104–107. [Google Scholar] [CrossRef]
- Inoue, T.; Komoda, H.; Uchida, T.; Node, K. Tropical fruit camu-camu (Myrciaria dubia) has anti-oxidative and anti-inflammatory properties. J. Cardiol. 2008, 52, 127–132. [Google Scholar] [CrossRef]
- Schwertz, M.C.; Maia, J.R.P.; de Sousa, R.F.S.; Aguiar, J.P.L.; Yuyama, L.K.O.; Lima, E.S. Efeito hipolipidêmico do suco de camu-camu em ratos. Rev. Nutr. 2012, 25, 35–44. [Google Scholar] [CrossRef]
- Nascimento, O.V.; Boleti, A.P.A.; Yuyama, L.K.O.; Lima, E.S. Effects of diet supplementation with Camu-camu (Myrciaria dubia HBK McVaugh) fruit in a rat model of diet-induced obesity. An. Acad. Bras. Cienc. 2013, 85, 355–363. [Google Scholar] [CrossRef]
- Gonçalves, A.E.S.S.; Lellis-Santos, C.; Curi, R.; Lajolo, F.M.; Genovese, M.I. Frozen pulp extracts of camu-camu (Myrciaria dubia McVaugh) attenuate the hyperlipidemia and lipid peroxidation of Type 1 diabetic rats. Food Res. Int. 2014, 64, 1–8. [Google Scholar] [CrossRef]
- Castro-Vargas, H.I.; Rodríguez-Varela, L.I.; Ferreira, S.R.S.; Parada-Alfonso, F. Extraction of phenolic fraction from guava seeds (Psidium guajava L.) using supercritical carbon dioxide and co-solvents. J. Supercrit. Fluids 2010, 51, 319–324. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Jiao, R.; Ma, K.Y. Cholesterol-Lowering Nutraceuticals and Functional Foods. J. Agric. Food Chem. 2008, 56, 8761–8773. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antwi, S.O.; Steck, S.E.; Zhang, H.; Stumm, L.; Zhang, J.; Hurley, T.G.; Hebert, J.R. Plasma carotenoids and tocopherols in relation to prostate-specific antigen (PSA) levels among men with biochemical recurrence of prostate cancer. Cancer Epidemiol. 2015, 39, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Moreda, W.; Pérez-Camino, M.; Cert, A. Gas and liquid chromatography of hydrocarbons in edible vegetable oils. J. Chromatogr. A 2001, 936, 159–171. [Google Scholar] [CrossRef]
- González-García, E.; Marina, M.L.; García, M.C. Plum (Prunus Domestica L.) by-product as a new and cheap source of bioactive peptides: Extraction method and peptides characterization. J. Funct. Foods 2014, 11, 428–437. [Google Scholar] [CrossRef]
- Xue, F.; Zhu, C.; Liu, F.; Wang, S.; Liu, H.; Li, C. Effects of high-intensity ultrasound treatment on functional properties of plum (Pruni domesticae semen) seed protein isolate. J. Sci. Food Agric. 2018, 98, 5690–5699. [Google Scholar] [CrossRef]
- González-García, E.; García, M.C.; Marina, M.L. Capillary liquid chromatography-ion trap-mass spectrometry methodology for the simultaneous quantification of four angiotensin-converting enzyme-inhibitory peptides in Prunus seed hydrolysates. J. Chromatogr. A 2018, 1540, 47–54. [Google Scholar] [CrossRef]
- Citadim, I.; Danner, M.A.; Sasso, S.A.Z. Rasileira de. Rev. Bras. Frutic. 2010, 32, 656. [Google Scholar]
- Jorge, N.; Luzia, D.M.M.; Bertanha, B.J. Antioxidant activity and profile fatty acids of jabuticaba seeds (Myrciaria cauliflora Berg). Acta Biol. Colomb. 2011, 16, 75–82. [Google Scholar]
- Baldin, J.C.; Munekata, P.E.S.; Michelin, E.C.; Polizer, Y.J.; Silva, P.M.; Canan, T.M.; Pires, M.A.; Godoy, S.H.S.; Fávaro-Trindade, C.S.; Lima, C.G.; et al. Effect of microencapsulated Jabuticaba (Myrciaria cauliflora) extract on quality and storage stability of mortadella sausage. Food Res. Int. 2018, 108, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.d.J.B.; Corrêa, A.D.; Dantas-Barros, A.M.; Nelson, D.L.; Amorim, A.C.L. Sugars, organic acids, minerals and lipids in jabuticaba. Rev. Bras. Frutic. 2011, 33, 540–550. [Google Scholar] [CrossRef] [Green Version]
- Batista, Â.G.; Lenquiste, S.A.; Moldenhauer, C.; Godoy, J.T.; Reis, S.M.P.M.; Maróstica Júnior, M.R. Jaboticaba (Myrciaria jaboticaba (Vell.) Berg.) peel improved triglycerides excretion and hepatic lipid peroxidation in high-fat-fed rats. Rev. Nutr. 2013, 26, 571–581. [Google Scholar] [CrossRef]
- Palozi, R.A.C.; Guarnier, L.P.; Romão, P.V.M.; Nocchi, S.R.; Dos Santos, C.C.; Lourenço, E.L.B.; Silva, D.B.; Gasparotto, F.M.; Gasparotto Junior, A. Pharmacological safety of Plinia cauliflora (Mart.) Kausel in rabbits. Toxicol. Rep. 2019, 6, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Dabas, D.; Shegog, R.; Ziegler, G.; Lambert, J. Avocado (Persea americana) Seed as a Source of Bioactive Phytochemicals. Curr. Pharm. Des. 2013, 19, 6133–6140. [Google Scholar] [CrossRef] [PubMed]
- Kosińska, A.; Karamać, M.; Estrella, I.; Hernández, T.; Bartolomé, B.; Dykes, G.A. Phenolic Compound Profiles and Antioxidant Capacity of Persea americana Mill. Peels and Seeds of Two Varieties. J. Agric. Food Chem. 2012, 60, 4613–4619. [Google Scholar] [CrossRef] [PubMed]
- Uchenna, U.E.; Shori, A.B.; Baba, A.S. Inclusion of avocado (Persea americana) seeds in the diet to improve carbohydrate and lipid metabolism in rats. Rev. Argent. Endocrinol. Metab. 2017, 54, 140–148. [Google Scholar] [CrossRef]
- Hatzakis, E.; Mazzola, E.P.; Shegog, R.M.; Ziegler, G.R.; Lambert, J.D. Perseorangin: A natural pigment from avocado (Persea americana) seed. Food Chem. 2019, 293, 15–22. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Poovarodom, S.; Leontowicz, H.; Leontowicz, M.; Vearasilp, S.; Trakhtenberg, S.; Gorinstein, S. The multiple nutrition properties of some exotic fruits: Biological activity and active metabolites. Food Res. Int. 2011, 44, 1671–1701. [Google Scholar] [CrossRef]
- Shanmugam, S.; Thangaraj, P.; Lima, B.d.S.; Chandran, R.; de Souza Araújo, A.A.; Narain, N.; Serafini, M.R.; Júnior, L.J.Q. Effects of luteolin and quercetin 3-β- d -glucoside identified from Passiflora subpeltata leaves against acetaminophen induced hepatotoxicity in rats. Biomed. Pharmacother. 2016, 83, 1278–1285. [Google Scholar] [CrossRef]
- Shanmugam, S.; Gomes, I.A.; Denadai, M.; dos Santos Lima, B.; de Souza Araújo, A.A.; Narain, N.; Neta, M.T.S.L.; Serafini, M.R.; Quintans-Júnior, L.J.; Thangaraj, P. UHPLC-QqQ-MS/MS identification, quantification of polyphenols from Passiflora subpeltata fruit pulp and determination of nutritional, antioxidant, α-amylase and α-glucosidase key enzymes inhibition properties. Food Res. Int. 2018, 108, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.M.; Sierra, C.A.; Rodríguez-Pulido, F.J.; González-Miret, M.L.; Heredia, F.J.; Osorio, C. Physicochemical characterisation of gulupa (Passiflora edulis Sims. fo edulis) fruit from Colombia during the ripening. Food Res. Int. 2011, 44, 1912–1918. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, Z.; Ramdath, D.D.; Tang, Y.; Chen, P.X.; Liu, R.; Liu, Q.; Tsao, R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on α-glucosidase and pancreatic lipase. Food Chem. 2015, 172, 862–872. [Google Scholar] [CrossRef]
- Gazola, A.C.; Costa, G.M.; Castellanos, L.; Ramos, F.A.; Reginatto, F.H.; de Lima, T.C.M.; Schenkel, E.P.; Gazola, A.C.; Costa, G.M.; Castellanos, L.; et al. Involvement of GABAergic pathway in the sedative activity of apigenin, the main flavonoid from Passiflora quadrangularis pericarp. Rev. Bras. Farmacogn. 2015, 25, 158–163. [Google Scholar] [CrossRef]
- Shuayprom, A.; Sanguansermsri, D.; Sanguansermsri, P.; Fraser, I.H.; Wongkattiya, N. Quantitative determination of vitexin in Passiflora foetida Linn. leaves using HPTLC. Asian Pac. J. Trop. Biomed. 2016, 6, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Saito, S.; Kawabata, J. Effects of electron-withdrawing substituents on DPPH radical scavenging reactions of protocatechuic acid and its analogues in alcoholic solvents. Tetrahedron 2005, 61, 8101–8108. [Google Scholar] [CrossRef]
- Lam, S.K.; Ng, T.B. Passiflin, a novel dimeric antifungal protein from seeds of the passion fruit. Phytomedicine 2009, 16, 172–180. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M. Antioxidant Activities and Phenolics of Passiflora edulis Seed Recovered from Juice Production Residue. J. Oleo Sci. 2013, 62, 235–240. [Google Scholar] [CrossRef]
- Malacrida, C.R.; Jorge, N. Yellow passion fruit seed oil (Passiflora edulis f. flavicarpa): Physical and chemical characteristics. Braz. Arch. Biol. Technol. 2012, 55, 127–134. [Google Scholar] [CrossRef]
- Macagnan, F.T.; dos Santos, L.R.; Roberto, B.S.; de Moura, F.A.; Bizzani, M.; da Silva, L.P. Biological properties of apple pomace, orange bagasse and passion fruit peel as alternative sources of dietary fibre. Bioact. Carbohydr. Diet. Fibre 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Helbig, D.; Böhm, V.; Wagner, A.; Schubert, R.; Jahreis, G. Berry seed press residues and their valuable ingredients with special regard to black currant seed press residues. Food Chem. 2008, 111, 1043–1049. [Google Scholar] [CrossRef]
- Pieszka, M.; Tombarkiewicz, B.; Roman, A.; Migdał, W.; Niedziółka, J. Effect of bioactive substances found in rapeseed, raspberry and strawberry seed oils on blood lipid profile and selected parameters of oxidative status in rats. Environ. Toxicol. Pharmacol. 2013, 36, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Destandau, E.; Le Floch, G.; Lucchesi, M.E.; Elfakir, C. Antimicrobial, antioxidant and phytochemical investigations of sea buckthorn (Hippophaë rhamnoides L.) leaf, stem, root and seed. Food Chem. 2012, 131, 754–760. [Google Scholar] [CrossRef]
- Sharma, U.K.; Sharma, K.; Sharma, N.; Sharma, A.; Singh, H.P.; Sinha, A.K. Microwave-Assisted Efficient Extraction of Different Parts of Hippophae rhamnoides for the Comparative Evaluation of Antioxidant Activity and Quantification of Its Phenolic Constituents by Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC) †. J. Agric. Food Chem. 2008, 56, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ahotupa, M.; Määttä, P.; Kallio, H. Composition and antioxidative activities of supercritical CO2-extracted oils from seeds and soft parts of northern berries. Food Res. Int. 2011, 44, 2009–2017. [Google Scholar] [CrossRef]
- Gustinelli, G.; Eliasson, L.; Svelander, C.; Andlid, T.; Lundin, L.; Ahrné, L.; Alminger, M. Supercritical Fluid Extraction of Berry Seeds: Chemical Composition and Antioxidant Activity. J. Food Qual. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Fazio, A.; Plastina, P.; Meijerink, J.; Witkamp, R.F.; Gabriele, B. Comparative analyses of seeds of wild fruits of Rubus and Sambucus species from Southern Italy: Fatty acid composition of the oil, total phenolic content, antioxidant and anti-inflammatory properties of the methanolic extracts. Food Chem. 2013, 140, 817–824. [Google Scholar] [CrossRef]
- Gođevac, D.; Tešević, V.; Vajs, V.; Milosavljević, S.; Stanković, M. Blackberry Seed Extracts and Isolated Polyphenolic Compounds Showing Protective Effect on Human Lymphocytes DNA. J. Food Sci. 2011, 76, C1039–C1043. [Google Scholar] [CrossRef]
- Van Hoed, V.; Barbouche, I.; De Clercq, N.; Dewettinck, K.; Slah, M.; Leber, E.; Verhé, R. Influence of filtering of cold pressed berry seed oils on their antioxidant profile and quality characteristics. Food Chem. 2011, 127, 1848–1855. [Google Scholar] [CrossRef]
- Parry, J.; Su, L.; Moore, J.; Cheng, Z.; Luther, M.; Rao, J.N.; Wang, J.-Y.; Yu, L.L. Chemical Compositions, Antioxidant Capacities, and Antiproliferative Activities of Selected Fruit Seed Flours. J. Agric. Food Chem. 2006, 54, 3773–3778. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Hartmann-Schmidlin, S.; Kähkönen, M.; Heinonen, M.; Määttä-Riihinen, K.; Oksman-Caldentey, K.M. Berry phenolics selectively inhibit the growth of intestinal pathogens. J. Appl. Microbiol. 2005, 98, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Bakowska-Barczak, A.M.; Schieber, A.; Kolodziejczyk, P. Characterization of Canadian Black Currant (Ribes nigrum L.) Seed Oils and Residues. J. Agric. Food Chem. 2009, 57, 11528–11536. [Google Scholar] [CrossRef] [PubMed]
- Puupponen-Pimiä, R.; Nohynek, L.; Juvonen, R.; Kössö, T.; Truchado, P.; Westerlund-Wikström, B.; Leppänen, T.; Moilanen, E.; Oksman-Caldentey, K.-M. Fermentation and dry fractionation increase bioactivity of cloudberry (Rubus chamaemorus). Food Chem. 2016, 197, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Hager, T.J.; Howard, L.R.; Liyanage, R.; Lay, J.O.; Prior, R.L. Ellagitannin Composition of Blackberry As Determined by HPLC-ESI-MS and MALDI-TOF-MS. J. Agric. Food Chem. 2008, 56, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Smeds, A.I.; Eklund, P.C.; Willför, S.M. Content, composition, and stereochemical characterisation of lignans in berries and seeds. Food Chem. 2012, 134, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food Chem. Toxicol. 2016, 97, 199–204. [Google Scholar] [CrossRef]
- Bal, L.M.; Meda, V.; Naik, S.N.; Satya, S. Sea buckthorn berries: A potential source of valuable nutrients for nutraceuticals and cosmoceuticals. Food Res. Int. 2011, 44, 1718–1727. [Google Scholar] [CrossRef]
- Basu, M.; Prasad, R.; Jayamurthy, P.; Pal, K.; Arumughan, C.; Sawhney, R.C. Anti-atherogenic effects of seabuckthorn (Hippophaea rhamnoides) seed oil. Phytomedicine 2007, 14, 770–777. [Google Scholar] [CrossRef]
- Yang, B.; Kortesniemi, M. Clinical evidence on potential health benefits of berries. Curr. Opin. Food Sci. 2015, 2, 36–42. [Google Scholar] [CrossRef]
- Yang, B.; Kalimo, K.O.; Mattila, L.M.; Kallio, S.E.; Katajisto, J.K.; Peltola, O.J.; Kallio, H.P. Effects of dietary supplementation with sea buckthorn (Hippophaë rhamnoides) seed and pulp oils on atopic dermatitis. J. Nutr. Biochem. 1999, 10, 622–630. [Google Scholar] [CrossRef]
- Larmo, P.S.; Kangas, A.J.; Soininen, P.; Lehtonen, H.-M.; Suomela, J.-P.; Yang, B.; Viikari, J.; Ala-Korpela, M.; Kallio, H.P. Effects of sea buckthorn and bilberry on serum metabolites differ according to baseline metabolic profiles in overweight women: A randomized crossover trial. Am. J. Clin. Nutr. 2013, 98, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.K.; Korte, H.; Yang, B.; Stanley, J.C.; Kallio, H.P. Sea buckthorn berry oil inhibits platelet aggregation. J. Nutr. Biochem. 2000, 11, 491–495. [Google Scholar] [CrossRef]
- Yang, B.; Kallio, H. Composition and physiological effects of sea buckthorn (Hippophaë) lipids. Trends Food Sci. Technol. 2002, 13, 160–167. [Google Scholar] [CrossRef]
- Linnamaa, P.; Nieminen, K.; Koulu, L.; Tuomasjukka, S.; Kallio, H.; Yang, B.; Tahvonen, R.; Savolainen, J. Black currant seed oil supplementation of mothers enhances IFN-γ and suppresses IL-4 production in breast milk. Pediatr. Allergy Immunol. 2013, 24, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Bhushan, B. An overview of the recent trends on the waste valorization techniques for food wastes. J. Environ. Manag. 2019, 233, 352–370. [Google Scholar] [CrossRef]
- Marathe, S.J.; Jadhav, S.B.; Bankar, S.B.; Kumari Dubey, K.; Singhal, R.S. Improvements in the extraction of bioactive compounds by enzymes. Curr. Opin. Food Sci. 2019, 25, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Fernández, K.; Vega, M.; Aspé, E. An enzymatic extraction of proanthocyanidins from País grape seeds and skins. Food Chem. 2015, 168, 7–13. [Google Scholar] [CrossRef]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C.F.R. Enzyme-assisted extractions of polyphenols—A comprehensive review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Pap, N.; Mahosenaho, M.; Pongrácz, E.; Mikkonen, H.; Jaakkola, M.; Virtanen, V.; Myllykoski, L.; Horváth-Hovorka, Z.; Hodúr, C.; Vatai, G.; et al. Effect of Ultrafiltration on Anthocyanin and Flavonol Content of Black Currant Juice (Ribes nigrum L.). Food Bioprocess Technol. 2012, 5, 921–928. [Google Scholar] [CrossRef]
- Fernandes, P.; Carvalho, F. Microbial enzymes for the food industry. In Biotechnology of Microbial Enzymes: Production, Biocatalysis and Industrial Applications, 1st ed.; Brahmachari, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 19, pp. 513–544. [Google Scholar]
- Nadar, S.S.; Rathod, V.K. Sonochemical Effect on Activity and Conformation of Commercial Lipases. Appl. Biochem. Biotechnol. 2017, 181, 1435–1453. [Google Scholar] [CrossRef] [PubMed]
- Roselló-Soto, E.; Galanakis, C.M.; Brnčić, M.; Orlien, V.; Trujillo, F.J.; Mawson, R.; Knoerzer, K.; Tiwari, B.K.; Barba, F.J. Clean recovery of antioxidant compounds from plant foods, by-products and algae assisted by ultrasounds processing. Modeling approaches to optimize processing conditions. Trends Food Sci. Technol. 2015, 42, 134–149. [Google Scholar] [CrossRef]
- Da Porto, C.; Porretto, E.; Decorti, D. Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.) seeds. Ultrason. Sonochem. 2013, 20, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Romdhane, M. Investigation in solid–liquid extraction: Influence of ultrasound. Chem. Eng. J. 2002, 87, 11–19. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Li, Y.; Skouroumounis, G.K.; Elsey, G.M.; Taylor, D.K. Microwave-assistance provides very rapid and efficient extraction of grape seed polyphenols. Food Chem. 2011, 129, 570–576. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Sovová, H.; Planinić, M.; Tomas, S. Temperature-dependent kinetics of grape seed phenolic compounds extraction: Experiment and model. Food Chem. 2013, 136, 1136–1140. [Google Scholar] [CrossRef]
- Herrero, M.; Castro-Puyana, M.; Rocamora-Reverte, L.; Ferragut, J.A.; Cifuentes, A.; Ibáñez, E. Formation and relevance of 5-hydroxymethylfurfural in bioactive subcritical water extracts from olive leaves. Food Res. Int. 2012, 47, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Duba, K.S.; Casazza, A.A.; Mohamed, H.B.; Perego, P.; Fiori, L. Extraction of polyphenols from grape skins and defatted grape seeds using subcritical water: Experiments and modeling. Food Bioprod. Process. 2015, 94, 29–38. [Google Scholar] [CrossRef]
- Plaza, M.; Marina, M.L. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2019, 116, 236–247. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Fathi, A.; Perego, P.; Dehghani, F. Extraction of antioxidants from winery wastes using subcritical water. J. Supercrit. Fluids 2012, 65, 18–24. [Google Scholar] [CrossRef]
- He, L.; Zhang, X.; Xu, H.; Xu, C.; Yuan, F.; Knez, Ž.; Novak, Z.; Gao, Y. Subcritical water extraction of phenolic compounds from pomegranate (Punica granatum L.) seed residues and investigation into their antioxidant activities with HPLC–ABTS+ assay. Food Bioprod. Process. 2012, 90, 215–223. [Google Scholar] [CrossRef]
- Monrad, J.K.; Suárez, M.; Motilva, M.J.; King, J.W.; Srinivas, K.; Howard, L.R. Extraction of anthocyanins and flavan-3-ols from red grape pomace continuously by coupling hot water extraction with a modified expeller. Food Res. Int. 2014, 65, 77–87. [Google Scholar] [CrossRef]
- Yilmaz, E.E.; Özvural, E.B.; Vural, H. Extraction and identification of proanthocyanidins from grape seed (Vitis Vinifera) using supercritical carbon dioxide. J. Supercrit. Fluids 2011, 55, 924–928. [Google Scholar] [CrossRef]
- Berna, A.; Cháfer, A.; Montón, J.; Subirats, S. High-pressure solubility data of system ethanol (1)+catechin (2)+CO2 (3). J. Supercrit. Fluids 2001, 20, 157–162. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A. Supercritical fluid extraction of polyphenols from grape seed (Vitis vinifera): Study on process variables and kinetics. J. Supercrit. Fluids 2017, 130, 239–245. [Google Scholar] [CrossRef]
- Ran, L.; Yang, C.; Xu, M.; Yi, Z.; Ren, D.; Yi, L. Enhanced aqueous two-phase extraction of proanthocyanidins from grape seeds by using ionic liquids as adjuvants. Sep. Purif. Technol. 2019, 226, 154–161. [Google Scholar] [CrossRef]
- Martins, P.L.G.; Braga, A.R.; de Rosso, V.V. Can ionic liquid solvents be applied in the food industry? Trends Food Sci. Technol. 2017, 66, 117–124. [Google Scholar] [CrossRef]
- Shi, J.; Nawaz, H.; Pohorly, J.; Mittal, G.; Kakuda, Y.; Jiang, Y. Extraction of Polyphenolics from Plant Material for Functional Foods—Engineering and Technology. Food Rev. Int. 2005, 21, 139–166. [Google Scholar] [CrossRef]
- Pap, N.; Pongrácz, E.; Jaakkola, M.; Tolonen, T.; Virtanen, V.; Turkki, A.; Horváth-Hovorka, Z.; Vatai, G.; Keiski, R.L. The effect of pre-treatment on the anthocyanin and flavonol content of black currant juice (Ribes nigrum L.) in concentration by reverse osmosis. J. Food Eng. 2010, 98, 429–436. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Caiazzo, F.; Drioli, E. Separation and purification of phenolic compounds from pomegranate juice by ultrafiltration and nanofiltration membranes. J. Food Eng. 2017, 195, 1–13. [Google Scholar] [CrossRef]
- Du, G.; Zhu, Y.; Wang, X.; Zhang, J.; Tian, C.; Liu, L.; Meng, Y.; Guo, Y. Phenolic composition of apple products and by-products based on cold pressing technology. J. Food Sci. Technol. 2019, 56, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Nile, A.; Liu, J.; Kim, D.H.; Kai, G. Exploitation of apple pomace towards extraction of triterpenic acids, antioxidant potential, cytotoxic effects, and inhibition of clinically important enzymes. Food Chem. Toxicol. 2019, 131, 110563. [Google Scholar] [CrossRef] [PubMed]
- Gunes, R.; Palabiyik, I.; Toker, O.S.; Konar, N.; Kurultay, S. Incorporation of defatted apple seeds in chewing gum system and phloridzin dissolution kinetics. J. Food Eng. 2019, 255, 9–14. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Ferreira, S.S.; Bastos, R.; Ferreira, I.; Cruz, M.T.; Pinto, A.; Coelho, E.; Passos, C.P.; Coimbra, M.A.; Cardoso, S.M.; et al. Apple Pomace Extract as a Sustainable Food Ingredient. Antioxidants 2019, 8, 189. [Google Scholar] [CrossRef]
- Rodríguez Madrera, R.; Suárez Valles, B. Characterization of apple seeds and their oils from the cider-making industry. Eur. Food Res. Technol. 2018, 244, 1821–1827. [Google Scholar] [CrossRef]
- Chandrasekar, V.; Martín-González, M.F.S.; Hirst, P.; Ballard, T.S. Optimizing Microwave-Assisted Extraction of Phenolic Antioxidants from Red Delicious and Jonathan Apple Pomace. J. Food Process Eng. 2015, 38, 571–582. [Google Scholar] [CrossRef]
- Zhang, Z.; Poojary, M.M.; Choudhary, A.; Rai, D.K.; Tiwari, B.K. Comparison of selected clean and green extraction technologies for biomolecules from apple pomace. Electrophoresis 2018, 39, 1934–1945. [Google Scholar] [CrossRef]
- Lohani, U.C.; Muthukumarappan, K. Application of the pulsed electric field to release bound phenolics in sorghum flour and apple pomace. Innov. Food Sci. Emerg. Technol. 2016, 35, 29–35. [Google Scholar] [CrossRef]
| Fruit Seed | Extracting Solvent | Total Phenolic Content | Individual Compounds | Bioactivity | Reference |
|---|---|---|---|---|---|
| Avocado (Persea americana) | Methanol/chloroform | not determined | Myristic acid Myristoleic Palmitic acid Palmitoleic acid Stearic acid Oleic acid Linoleic acid α-Linolenic acid Eicosadienoic acid Eicosatrienoic acid | DPPH radical scavenging activity ABTS scavenging assay Anti-inflammatory activity Anticancer activity (HepG2 and HCT116) | [51] |
| Hydroalcoholic 70% (ethanol) | 366.79 ± 5.05 mg GAE/g | Palmitic acid Linoleic acid Oleic acid Stearic acid p-Hydroxycoumaroyl quinic acid Caffeoylquinic acid Feruloylquinic acid | Gastroprotective activity | [61] | |
| Ethanol-water (1:1, v/v) | not determined | Vanillic acid 3-Feruloylquinic acid 5-O-caffeoylquinic acid Procyanidin dimer B7 Procyanidin pentamer B1 Procyanidin trimer A4 4-O-caffeoylquinic acid Procyanidin trimer B2 Procyanidin tetramer B1 Procyanidin dimer B8 Procyanidin pentamer B2 Procyanidin trimer A5 Benzoic acid Procyanidin dimer B9 p-Coumaric acid Procyanidin dimer A1 (−)-Epicatechin (Epi)catechin gallate 5-p-Coumaroylquinic acid Vanillin 4-p-Coumaroylquinic acid Pyrocatechol Procyanidin trimer A1 Procyanidin dimer B3 Syringic acid Procyanidin dimer B4 Procyanidin trimer B1 Tyrosol-glucoside Ferulic acid Procyanidin trimer A2 (Epi)catechin glucopyranoside (isomer 2) 4-Hydroxybenzoic acid Procyanidin tetramer A4 Procyanidin dimer B5 (+)-Catechin Hydroxyferulic acid 3-p-Coumaroylquinic acid Gentisic acid Procyanidin trimer A3 Tyrosol-glucosyl-pentoside Procyanidin tetramer A5 Penstemide Procyanidin dimer B Procyanidin dimer B10 Procyanidin trimer A6 Dihydrocaffeic acid Procyanidin dimer A2 Cinchonain (isomer 1) Caffeic acid 4-Feruloylquinic acid Procyanidin dimer A3 Procyanidin trimer A7 Procyanidin dimer A4 Procyanidin dimer B11 Quercetin-diglucoside (isomer 1) Quercetin-diglucoside (isomer 2) Procyanidin dimer A5 Hydroxyabscisic acid glucoside (Epi)gallocatechin Quercetin-3-β-glucoside Ethyl Protocatechuate Cinchonain (isomer 2) Quercetin (±)-Naringenin Sakuranetin | Trolox equivalent antioxidant capacity (TEAC) ABTS scavenging assay DPPH radical scavenging activity | [60] | |
| Aqueous methanol 80% and aqueous acetone 70% | 328.8 ± 13.5 mg GAE/g | Caffeic acid (−)-Epicatechin Vanillin p-Coumaric acid Ferulic acid Sinapic acid Procyanidin B4 Quercetin diglucoside Quercetin-3-O-arabinosyl-glucoside Quercetin-3-O-glucoside Quercetin-3-O-rutinoside Quinic acid Procyanidin dimer A Procyanidin trimer B1 Procyanidin dimer B1 Procyanidin trimer B2 Syringic acid Procyanidin dimer B2 (+)-Catechin Procyanidin trimer A Procyanidin dimer B2 Procyanidin trimer B3 5-O-Caffeoyl-quinic acid Quercetin Phloridzin Quercetin 3-O-rhamnoside Quercetin Apigenin Kaempferol | ABTS scavenging assay DPPH radical scavenging activity | [59] | |
| Water | 45 mg GAE/L | - | ORAC (oxygen radical antioxidant capacity) | [62] | |
| Grape (Vitis sp.) | Hydroalcoholic 60% (ethylic alcohol) | 13643±690 mg GAE/g | Delphinidin-3-glucoside Malvidin-3-glucoside Cyanidin-3-glucoside Malvidin-3,5-diglucoside 2,5 Dihydroxybenzoic acid 2,4 Dihydroxybenzoic acid Syringic acid 5-O-Caffeoylquinic acid Gallic acid 2-Hydroxycynnamic acid Ellagic acid Quercetin Quercetin-3-rutinoside (+)-Catechin (-)-Epicatechin trans-Resveratrol | Ferric-reducing antioxidant power (FRAP) Folin-Ciocalteu reducing capacity DPPH radical scavenging activity | [64] |
| Ethanol/acidic water (pH 3.2) 7:3 v/v | not determined | Vanillic acid p-Coumaric acid E-resveratrol Quercetin Kaempferol Pinoresinol | PTP-1B (Protein Tyrosine Phosphatase 1B enzyme) inhibitory power | [28] | |
| Ethanol/water (80:20, v/v) | 161.66 mg GAE/g | Phenolic acid Gallic acid Epicatechin Epigallocatechin gallate Epicatechin gallate Epigallocatechin Procyanidin B1 Procyanidin B4 Procyanidin B2 Flavonol Kaempferol Myricetin Quercetin | Cytotoxicity in murine macrophage RAW 264.7 Anti-inflammatory activity | [65] | |
| 50 mM acetate buffer at 1:10 w/v | 0.81 g GAE/100g | Gallic acid p-Coumaric acid Syringic acid (+)-Catechin Resveratrol Malvidin-3-O-glucoside | TEAC | [68] | |
| Passion fruit (Passiflora sp) | Acetonitrile | ~40 mg GAE/100g | 4-hydroxybenzoic acid Chlorogenic acid Vanillic acid Caffeic acid p-Coumaric acid Ferulic acid Rutin Quercetin trans-Cinnamic acid | ABTS scavenging assay DPPH radical scavenging activity Gastroprotective activity | [70] |
| Water | 0.14 mg GAE/mL | Vanillic acid Syringic acid Gallic acid Rutin Quercetin | ORAC and DPPH assays Folin-Ciocalteu assay | [66] | |
| Hexane Ethyl acetate Ethanol Ethanol/water | Maceration (EtOH–H2O 142.4 ± 0.4 mg GAE/g, EtOH 75 ± 2 mg GAE/g, EtOAc 24.6 ± 0.8 mg, GAE/g, Hx 30 ± 1 mg GAE/g) Ultrasound-assisted (EtOH–H2O 61.3 ± 0.4 mg GAE/g, EtOH 21.4 ± 0.4 mg GAE/g, EtOAc 19 ± 1 mg GAE/g, Hx 22.3 ± 0.1 mg GAE/g) Supercritical fluid extraction (40 °C 150 bar 33.0 ± 0.8 mg GAE/g, 250 bar 30 ± 1 mg GAE/g, 300 bar 18 ± 2 mg GAE/g, 50 °C 150 bar 26.4 ± 0.8 mg GAE/g, 250 bar 24.3 ± 0.4 mg GAE/g, 300 bar 19.3 ± 0.8 mg GAE/g) | not determined | ABTS scavenging assay DPPH radical scavenging activity Antimicrobial activity | [69] | |
| Petroleum ether Chloroform Acetone Methanol | 115.13 ± 8.42 mg GAE/g, 113.45 ± 6.19 mg GAE/g, 417.65 ± 7.33 mg GAE/g, 227.17 ± 10.97 mg GAE/g | Quercetin Gallic acid Apigenin Catechin | DPPH FRAP Metal chelating activity Superoxide radical scavenging activity Analgesic activity Anti-inflammatory activity Antipyretic effect | [67] | |
| Pomegranate (Punica granatum) | Ethanolic extracts - soluble and insoluble-bound phenolic compounds | 3.1 ± 0.3 (mg GAE/g) | not determined | ORAC and TEAC assays | [41] |
| Ethanolic extracts | 73 ± 13.35 (mg GAE/g) | not determined | Folin-Ciocalteu reducing capacity and β-Carotene oxidation method | [42] | |
| Methanolic extracts (oil) | 88.45 ± 3.89 mg GAE/kg oil | not determined | DPPH | [43] | |
| Isolation of free, esterified and insoluble-bound phenolic compounds | Free 1.38 ± 0.01 Esterified 1.39 ± 0.01 and Insoluble-bound 0.62 ± 0.01 (mg GAE/g) | Gallic acid (major phenolic acid present) | TEAC, electron paramagnetic resonance (EPR) spectrometry, Metal chelating ability | [44] | |
| Ethanolic extracts (oil) | not determined | Palmitic (16:0) stearic (18:0) oleic (18:1 ω-9) linoleic (18:2 ω-6) acids | β-carotene bleaching assays, DPPH, ORAC, and ABTS assays | [45] | |
| Camu-camu (Myrciaria dubia) | aqueous acetone (50:50 v/v) | 369.4±9.6 (mg GAE/g) | not determined | ABTS scavenging assay DPPH radical scavenging activity | [46] |
| Ethanolic extracts | 3738.0 ± 20.8 mg GAE/100 g | Ellagic acid Syringic acid Quercetin Myricetin Catechin | DPPH, Folin–Ciocalteau reducing capacity | [47] | |
| Aqueous extracts | not detected | not determined | FRAP and DPPH assays | [48] | |
| Ethanolic extracts | 128 mg GAE/100 g | Rosmarinic acid 2,4-dihydroxybenzoic acid Ellagic acid Cyanidin-3-glucoside Methylvescalagin trans-Resveratrol Quercetin | DPPH, FRAP and Folin-Ciocalteu reducing capacity | [49] | |
| EtOH: ethyl alcohol, H2O: water, (CH3)2CO: propanone) | 2400 (EtOH), 4000 (H2O) and 1300 ((CH3)2CO) mg GAE/100 g | Gallic and chlorogenic acid (H2O) Ellagic acid (EtOH) Ferulic acid ((CH3)2CO) | DPPH, FRAP and Folin-Ciocalteu reducing capacity assays | [15] | |
| Jabuticaba (Plinia jaboticaba and Plinia cauliflora) | Ethanolic extracts | 116.17 ± 7.10 mg of GAE/ g | not determined | not determined | [53] |
| Soluble phenolics, Alkaline hydrolysis and Acid hydrolysis | not determined | Cyanidin-3-O-glucoside Gallic acid Delphinidin-3-O-glucoside Rutin | Folin–Ciocalteu, FRAP, TEAC and ORAC assays | [54] | |
| water:propanone (60:40 v/v) | 8.65 g GAE/100 g | Ellagic acid and ellagitannins | DPPH scavenging activity and total reducing capacity | [55] | |
| Methanolic extracts | not determined | Ellagic acid derivatives | Folin–Ciocalteu reducing capacity, DPPH and FRAP assays | [56] | |
| Apple (Malus domestica) | Methanol | 5.74–17.44 GAE/g | Protocatechuic acid (+)-Catechin Proanthocyanin B2 Chlorogenic acid Caffeic acid (-)-Epicatechin Quercetin Hyperin Phloridzin | DPPH, FRAP and ABTS assays | [50] |
| Aqueous acetone (30:70; v/v) and ethyl acetate extraction after hexane extraction | not determined | 3-p-Coumaroylquinic acid 5-Caffeoylquinic acid 4-Caffeoylquinic acid Caffeic acid 3-Caffeoylquinic acid 5-p-Coumaroylquinic acid 4-p-Coumaroylquinic acid Proanthocyanin B2 Epicatechin 3-hydroxyphloridzin Phloretin-xyloglucoside Phloridzin Phloretin Procyanidins | not determined | [52] | |
| Ethanol-water (30:70) | not determined | Phloridzin | not determined | [58] | |
| Methanol | not determined | Quercetin Quercetin-3-O-galactoside Quercetin-3-O-glucoside Quercetin-3-O-rhamnoside Phloridzin Phloretin (+)-Catechin (-)-Epicatechin Cyanidin-3-O-galactoside Luteolin-7-O-glucoside Caffeic acid Chlorogenic acid Ferulic acid Isoferulic acid | not determined | [57] | |
| Methanol | not determined | Epicatechin Procyanidin B2 Catechin Chlorogenic acid p-Coumaroylquinic acid Quercetin-3-O-galactoside Quercetin-3-O-rhamnoside Quercetin-3-O-glucoside Phloridzin Phloretin-xyloglucoside Phloretin | not determined | [71] | |
| Hexane and 70% aqueous acetone after hexane extraction | not determined | Linoleic acid Palmitic acid Linolenic acid Stearic acid Oleic acid Chlorogenic acid p-Coumaroylquinic acid Quercetin-3-galactoside Quercetin-3-rutinoside Quercetin-3-glucoside 3-hydroxyphloridzin Quercetin-3-xyloside Phloretin-2′-xyloglucoside Quercetin-3-arabinoside Quercetin-3-rhamnoside Phloridzin | not determined | [72] | |
| Plum (Prunus sp.) | Aqueous methyl alcohol (50%) and aqueous acetone (70%) | Total extractable polyphenols 86 mg gallic acid/100 g | ABTS assay | [63] | |
| Total carotenoids 3.9 μg/g | |||||
| n-hexane and ultrasonication | α-Tocopherol | [73] | |||
| β-Tocopherol | |||||
| γ-Tocopherol | |||||
| δ-Tocopherol | |||||
| α-Tocotrienol | |||||
| γ-Tocotrienol | |||||
| Cholesterol | |||||
| Campesterol | |||||
| Δ5-Stigmasterol | |||||
| β-Sitosterol | |||||
| Sitostanol | |||||
| Δ5-Avenasterol | |||||
| Δ7-Stigmasterol | |||||
| Cycloartenol |
| Fruit Seeds | Extraction Solvents | Total Phenolics a | Individual Phenolics | Bioactivity b | Reference |
|---|---|---|---|---|---|
| Bilberry | |||||
| Vaccinium myrtillus | water | 2.3 mg GAE/g dry extract | not determined | Anti-oxidative activity TEAC (0.1 and 82 μmol TE/g dry extract in n-hexane and distilled water, respectively) | [159] |
| seed oil, Vaccinium myrtillus | not determined | not determined | Anti-oxidative activity OOR• scavenging (519 μmol α-tocopherol equivalent/100 g oil), Inhibition of microsomal lipid peroxidation (1188 μmol trolox equivalent/100 g oil) | [163] | |
| seed oil, Vaccinium myrtillus | not determined | not determined | Antioxidant activity DPPH (EC50 = 5.5–9.5 mg oil/mL) | [164] | |
| Blackberry | |||||
| Rubus spp. | methanol:acetone:water (7:7:6, v/v/v), acetone:water (8:2, v/v), methanol:water (7:3, v/v), water, diethyl ether:ethyl acetate (1:1, v/v) | 13 mg GAE/g dry extract | protocatechuic acid, p-coumaric acid, gallic acid, gallic hexoside, caffeic acid, syringic acid, catechin, epicatechin, epigallocatechin, epicatechin gallate, B-type procyanidin dimmers, quercetin, quercetin 3-O-glucoronide, quercetin pentose, myricetin, peonidin 3-O-glucoside | Antioxidant activity OH• scavenging (125 μmol TE/g dry extract), ORAC (60 μmol TE/g dry extract), Reducing power (88 μmol TE/g dry extract), Inhibition against human LDL-cholesterol oxidation (21–60% from 0 to 22 h), Iron(II) chelating (199 μmol TE/g dry extract), Inhibition of OH• and OOR• induced supercoiled DNA strand scission (85–95% for OH•, and 90–95% for OOR•) | [19] |
| Rubus ulmifolius | methanol | 171 mg GAE/g dry seeds | cyanidin 3-O-glucoside, ellagic acid, galloyl-HHDP-glucose, galloyl-bis-HHDP-glucose | Antioxidant activity DPPH (93, 96, and 98% radicals scavenged at 42, 83, 167 μg/mL of extracts, respectively; EC50 = 1 μg/mL) Anti-inflammatory activity Strong inhibitory effects on the production of LPS-induced inflammatory mediators (NO, CCL-20) | [165] |
| Rubus fruticosus 3 cultivars | methanol:water (1:1, v/v) | not determined | Ellagitannins (41 compounds), ellagic acid derivatives (10), gallic acid derivatives (4), protocatechuic acid, chlorogenic acid, salicylic acid | Protective effect on chromosome aberrations in peripheral human lymphocytes | [166] |
| seed oil, Rubus fruticosus | methanol:water (1:1, v/v) | 5.6–9.1 mg CAE/g oil | p-coumaric acid, vanillic acid, vanillin | Antioxidant activity FRAP (0.3 FeSO4 equivalent (μmol/L)/g oil) | [167] |
| Blueberry | |||||
| Vaccinium spp. | methanol:acetone:water (7:7:6, v/v/v), acetone:water (8:2, v/v), methanol:water (7:3, v/v), water, diethyl ether:ethyl acetate (1:1, v/v) | 2 mg GAE/g dry extract | protocatechuic acid, p-coumaric acid, gallic acid, gallic hexoside, caffeic acid, syringic acid, catechin, epicatechin, epigallocatechin, epicatechin gallate, B-type procyanidin dimmers, quercetin, quercetin pentose, myricetin, kaempferol hexoside, delphinidin 3-O-hexoside, cyanidin 3-O-(6-O-acetyl)galactoside, peonidin 3-O-glucoside, peonidin 3-O-arabinoside, petunidin 3-O-galactoside, petunidin 3-O-arabinoside | Antioxidant activity OH• scavenging (37 μmol TE/g dry extract), ORAC (5 μmol TE/g dry extract), Reducing power (60 μmol TE/g dry extract), Inhibition against human LDL-cholesterol oxidation (6–49% from 0 to 22 h), Iron(II) chelating (12 μmol TE/g dry extract), Inhibition of OH• and OOR• induced supercoiled DNA strand scission (50–70% for OH•, and 55–75% for OOR•) | [19] |
| seed oil, Vaccinium corymbosum | methanol:water (1:1, v/v) | 8.8–9.5 mg CAE/g oil | homovanillic acid, vanillin | Antioxidant activity FRAP (0.26–0.41 FeSO4 equivalent (μmol/L)/g oil) | [167] |
| seed flour, Vaccinium corymbosum | acetone:water (1:1, v/v) | 16 mg GAE/g seed flour | not determined | Antioxidant activity ORAC (153 μmol TE/g seed flour), DPPH (ED50 = 670 μg flour equivalents/mL), Iron(II) chelating (1.9 mg EDTA equivalents/g seed flour) | [168] |
| Cloudberry | |||||
| Rubus chamaemorus, after fermentation | acetone:water (7:3, v/v) | 457 mg/g dry extracts (measured by HPLC) | ellagic acid, ellagic acid glycosides, sanguiin H10, casuarictin/potentillin, lambertianin C, sanguiin H6, sanguiin H2, ferulic acid, quercetin 3-O-[6″-(3-hydroxy- 3-methylglutaroyl)-β-glucoside], quercetin-3-O-glucuronide | Anti-bacterial activity Staphylococcus aureus (very strong inhibition), Escherichia coli (strong inhibition), Pseudomonas aeruginosa (clear inhibition), Candida albicans (no inhibitory effects), Saccharomyces cerevisiae (no inhibitory effects) Anti-inflammatory activity significantly reduced NO and IL-6 production and iNOS expression in activated macrophages | [171] |
| seed oil, Rubus chamaemorus | not determined | not determined | Antioxidant activity OOR• scavenging (1157 μmol α-tocopherol equivalent/100 g oil), Inhibition of microsomal lipid peroxidation (650 μmol trolox equivalent/100 g oil) | [163] | |
| Cranberry | |||||
| Vaccinium oxycoccus | water | not determined | not determined | Antioxidant activity TEAC (0.1 and 50 μmol TE/g dry extract in n-hexane and distilled water, respectively) | [159] |
| seed oil, Vaccinium macrocarpon | methanol:water (1:1, v/v) | 11.0–11.3 mg CAE/g oil | 4-(2-hydroxyethyl)phenol, p-coumaric acid, homovanillic acid, vanillic acid, protocatechuic acid | Antioxidant activity FRAP (0.27–0.29 FeSO4 equivalent (μmol/L)/g oil) | [167] |
| seed flour, Vaccinium macrocarpon | acetone:water (1:1, v/v) | 15 mg GAE/g seed flour | not determined | Antioxidant activity ORAC (111 μmol TE/g seed flour), DPPH (ED50 = 1260 μg flour equivalents/mL), Iron(II) chelating (2.1 mg EDTA equivalents/g seed flour) Anti-proliferative activity significant inhibited HT-29 cell proliferation | [168] |
| seed oil, Vaccinium oxycoccos | not determined | not determined | Antioxidant activity OOR• scavenging (1543 μmol α-tocopherol equivalent/100 g oil), Inhibition of microsomal lipid peroxidation (399 μmol trolox equivalent/100 g oil) | [163] | |
| seed oil, Vaccinium macrocarpon | not determined | not determined | Antioxidant activity OOR• scavenging (975 μmol α-tocopherol equivalent/100 g oil), Inhibition of microsomal lipid peroxidation (338 μmol trolox equivalent/100 g oil) | [163] | |
| Currant | |||||
| black, Ribes nigrum 5 cultivars | acetone:water (1:1, v/v) | 1.6–2.3 mg GAE/g dry seed residues | delphinidin 3-O-glucoside, delphinidin 3-O-rutinoside, cyanidin 3-O-glucoside, cyanidin 3-O-rutinoside, myricetin 3-O-glucoside, myricetin 3-O-rutinoside, quercetin 3-O-glucoside, quercetin 3-O-rutinoside, kaempferol 3-O-glucoside, kaempferol 3-O-rutinoside, p-coumaric acid, p-coumaroyl glycoside | Antioxidant activity ABTS (14–17 μmol TE/g dry seed residues), DPPH (11–13 μmol TE/g dry seed residues) | [170] |
| black, Ribes nigrum | water | 0.9–1.8 mg GAE/g dry extract | not determined | Antioxidant activity TEAC (0.6–0.7 and 26–60 μmol TE/g dry extract in n-hexane and distilled water, respectively) | [159] |
| black, seed oil, Ribes nigrum | not determined | not determined | Antioxidant activity OOR• scavenging (1068 μmol α-tocopherol equivalent/100 g oil), Inhibition of microsomal lipid peroxidation (478 μmol trolox equivalent/100 g oil) | [163] | |
| Elderberry | |||||
| Sambucus nigra | methanol | 54 mg GAE/g dry seeds | cyaniding 3-O-sambubioside -5-O-glucoside, pelargonidin 3-O-rutinoside, quercetin 3-O-rutinoside, quercetin 3-O-glucoside | Antioxidant activity DPPH (42, 55, 82% radicals scavenged at 42, 83, 167 μg/mL of extracts, respectively; EC50 = 82 μg/mL) Anti-inflammatory activity No inhibitory effects on the production of LPS-induced inflammatory mediators (NO, CCL-20) | [165] |
| Sambucus nigra | water | 1.5 mg GAE/g dry extract | not determined | Antioxidant activity TEAC (0.8 and 30 μmol TE/g dry extract in n-hexane and distilled water, respectively) | [159] |
| Lingonberry | |||||
| seed oil, Vaccinium vitis-idaea | not determined | not determined | Antioxidant activity OOR• scavenging (299 μmol α-tocopherol equivalent/100 g oil), Inhibition of microsomal lipid peroxidation (329 μmol trolox equivalent/100 g oil) | [163] | |
| Raspberry | |||||
| black, Rubus spp. | methanol:acetone:water (7:7:6, v/v/v), acetone:water (8:2, v/v), methanol:water (7:3, v/v), water, diethyl ether:ethyl acetate (1:1, v/v) | 7 mg GAE/g dry extract | protocatechuic acid, p-coumaric acid, gallic acid, gallic hexoside, caffeic acid, syringic acid, catechin, epicatechin, epigallocatechin, epicatechin gallate, B-type procyanidin dimmers, quercetin, quercetin 3-O-glucoronide, quercetin pentose, myricetin, peonidin 3-O-glucoside | Antioxidant activity OH• scavenging (67 μmol TE/g dry extract), ORAC (47 μmol TE/g dry extract), Reducing power (60 μmol TE/g dry extract), Inhibition against human LDL-cholesterol oxidation (18–57% from 0 to 22 h), Iron(II) chelating (69 μmol TE/g dry extract), Inhibition of OH• and OOR• induced supercoiled DNA strand scission (60-80% for OH•, and 80-85% for OOR•) | [19] |
| seed oil, Rubus spp. | methanol | 2.7 mg CAE/100 g oil | not determined | Antioxidant activity Inhibitory effects on the activities of superoxide dismutase and glutathione peroxidase | [160] |
| red, seed oil, Rubus idaeus | methanol:water (1:1, v/v) | 8.4 mg CAE/g oil | 4-(2-hydroxyethyl)phenol | Antioxidant activity FRAP (0.34 FeSO4 equivalent (μmol/L)/g oil) | [167] |
| black, seed flour, Rubus occidentalis | acetone:water (1:1, v/v) | 41 mg GAE/g seed flour | not determined | Antioxidant activity ORAC (296 μmol TE/g seed flour), DPPH (ED50 = 200 μg flour equivalents/mL), Iron(II) chelating (3.6 mg EDTA equivalents/g seed flour) Anti-proliferative activity significant inhibited HT-29 cell proliferation | [168] |
| red, seed flour, Rubus ideaus | acetone:water (1:1, v/v) | 25 mg GAE/g seed flour | not determined | Antioxidant activity ORAC (276 μmol TE/g seed flour), DPPH (ED50 = 510 μg flour equivalents/mL), Iron(II) chelating (3.9 mg EDTA equivalents/g seed flour) | [168] |
| seed oil, Rubus idaeus | not determined | not determined | Antioxidant activity OOR• scavenging (2315 μmol α-tocopherol equivalent/100 g oil), Inhibition of microsomal lipid peroxidation (424 μmol trolox equivalent/100 g oil) | [163] | |
| Sea buckthorn | |||||
| Hippophaë rhamnoides | ethanol:water (1:9, v/v) | 120 mg GAE/g dry extract | tannins, gallic acid, quercetin 3-O-galactoside, kaempferol, isorhamnetin, isorhamnetin 3-O-glucoside, isorhamnetin 3-O-rutinoside | Antioxidant activity DPPH (529 mg TE/g dry extract), FRAP (454 mg TE/g dry extract) Anti-bacterial activity Enterecoccus durans (68% inhibition percentage), Candida albicans (68%), Bacillus cereus (64%), Staphylococcus aureus (41%), Escherichia coli (38%), Pseudomonas aeruginosa (28%) | [161] |
| Hippophaë rhamnoides | ethanol | 9.4–23.5 mg GAE/g dry seeds | quercetin-3-O-galactoside, quercetin, myricetin, isorhamnetin | Antioxidant activity ABTS (44–182 μmol TE/g dry seeds), DPPH (128–283 μmol TE/g dry seeds) | [162] |
| seed oil, Hippophaë rhamnoides | not determined | not determined | Antioxidant activity OOR• scavenging (1323 μmol α-tocopherol equivalent/100 g oil), Inhibition of microsomal lipid peroxidation (245 μmol trolox equivalent/100 g oil), Inhibition of LDL oxidation (IC50 = 2.0 μL oil/mg LDL), O2•- scavenging (IC50 = 2 g oil/L), Inhibition against DNA oxidation (protective effects on purified DNA and rat liver homogenate from UV-induced DNA oxidation in vitro) | [163] | |
| Strawberry | |||||
| seed oil, Fragaria × ananassa | methanol | 1.8 mg CAE/100 g oils | not determined | Antioxidant activity Inhibitory effects on the activities of superoxide dismutase and glutathione peroxidase | [160] |
| Fragaria × ananassa | water | 1.7 mg GAE/g dry extract | not determined | not determined | [159] |
| seed oil, Fragaria × ananassa | methanol:water (1:1, v/v) | 9.3–10.4 mg CAE/g oil | p-coumaric acid, ferulic acid, 4-(2-hydroxyethyl)phenol, homovanillic acid, vanillic acid, vanillin. | Antioxidant activity FRAP (0.24–0.30 FeSO4 equivalent (μmol/L)/g oil) | [167] |
| seed oil, Fragaria × ananassa | not determined | not determined | Antioxidant activity OOR• scavenging (296 μmol α-tocopherol equivalent/100 g oil), Inhibition of microsomal lipid peroxidation (1015 μmol trolox equivalent/100 g oil) | [163] |
| Extraction | Agro-Industrial Waste | Total Bioactive Compounds | Individual Bioactive Compounds | Antioxidant Activity (AA) | Conclusion Remarks | Reference |
|---|---|---|---|---|---|---|
| Conventional extraction: 30 °C for 30 min in a shaker 70% with methanol containing 2% formic acid | Apple pomace (peel and seed) | Total phenols (mg GAE/L) Peel: 2228.49 Pomace: 208.75 Total flavanols (mg CTE/L) Peel: 170.33 Pomace: 40.91 | Phenolic compounds (mg/L) Peel: Gallic acid: 40.42 Vanillic acid: 58.70 Caffeic acid: 0.21 Chlorogenic acid: 7.05 Catechin: 37.67 Epicatechin gallate: 12.85 Phlorizin: 60.28 Pomace: Gallic acid: 29.57 Vanillic acid: 56.02 Chlorogenic acid: 5.29 Catechin: 35.40 Epicatechin gallate: 21.68 Phlorizin: 12.81 | DPPH (µmol TE/L) Peels: 4203.59 Pomace: 3830.72 | Peel pomace contained significantly higher amounts of bioactive compounds in comparison to pulp pomace. Due to high levels of antioxidant and bioactive compounds, apple pomaces could be a better material to make apple cider vinegar. | [211] |
| Conventional extraction: Magnetic stirring with methanol during 30 min. After centrifugation and filtration, methanol was evaporated through Soxhlet extraction (60 ± 0.5 °C). Crude extract was further dissolved and extracted with ethanol, chloroform, ethyl acetate and n-hexane to obtain their fractions. | Apple pomace freeze-dried and milled | Total phenols (mg GAE/g DW) Methanol: 3.48 Ethanol: 3.04 Ethyl acetate: 2.85 Chloroform: 1.64 n-hexane: 1.50 | The average amounts of 6 triterpenoic acids detected (mg/100 g DW): maslinic acid: 0.96 oleanolic acid: 3.18 ursolic acid: 6.14 betulinic acid: 1.78 erythrodiol: 0.78 uvaol: 0.84 | DPPH (%) Methanol: 72.6 Ethanol: 67.5 Ethyl acetate: 64.2 Chloroform: 59.6 n-hexane: 56.2 FRAP (%) Methanol: 65.8 Ethanol: 60.3 Ethyl acetate: 58.3 Chloroform: 55.2 n-hexane: 50.1 ABTS (%) Methanol: 84.3 Ethanol: 79.2 Ethyl acetate: 72.9 Chloroform: 65.3 n-hexane: 60.8 | The antioxidant activity results showed that the methanol and ethanol fractions were more effective radical scavengers than chloroform, ethyl acetate and n-hexane. Both alcoholic fractions showed potential toward tyrosinase, xanthine oxidase and urease inhibition. Among the TAAs, ursolic acid, betulinic acid and maslinic acid showed effective radical scavenging activity. All TAAs showed lower inhibitory activity towards free radicals in vitro than the apple pomace extracts. Apple pomace methanol extract and ursolic acid revealed prominent anticancer activity on Hela, Skov-3, Caski, and NCL cancer cell lines, respectively. | [212] |
| Ultrasound Assisted Extraction (UAE): Defatted seed flours were mixed with 80% methanol and held in an ultrasonic bath for 30 min at room temperature. | Apple seeds (defatted seed flours) | Total phenols (mg GAE/kg DS) Fuji Zhen Aztec 2861 Granny Smith 3581 Jeromine 4096 Pink Lady 3616 Super Chief 5141 | Phenolic compounds (mg/kg DM): Fuji Zhen Aztec Phloridzin: 1748.7 Ellagic acid: 189.5 (-)-Epicatechin: 76.3 Caffeic acid: 114.2 (+)-Catechin: 90.1 Ferulic acid: 57.8 Protocatechuic acid: 46.8 Gallic acid: 4.2 Granny Smith Phloridzin: 2106.6 Ellagic acid: 275.3 (-)-Epicatechin: 77.2 Caffeic acid: 9.1 (+)-Catechin: 5.0 Ferulic acid: 142.2 Protocatechuic acid: 48.7 Gallic acid: 7.5 Jeromine Phloridzin: 2623.5 Ellagic acid: 286.7 (-)-Epicatechin: 164.6 Caffeic acid: 11.8 (+)-Catechin: N.D. Ferulic acid: 21.2 Protocatechuic acid: 153.8 Gallic acid: 5.6 Pink Lady Phloridzin: 1888.4 Ellagic acid: 216.5 (-)-Epicatechin: 73.9 Caffeic acid: 10.4 (+)-Catechin: 21.0 Ferulic acid: 8.2 Protocatechuic acid: 39.6 Gallic acid: 4.2 Super Chief Phloridzin: 3462.2 Ellagic acid: 230.7 (-)-Epicatechin: 69.0 Caffeic acid: 14.2 (+)-Catechin: 191.0 Ferulic acid: 36.4 Protocatechuic acid: 161.3 Gallic acid: 7.9 | ABTS (mg GAE/kg DS) Fuji Zhen Aztec 363 Granny Smith 292 Jeromine 368 Pink Lady 392 Super Chief 343 mg DPPH µmol TE/g DS) Fuji Zhen Aztec 24.28 Granny Smith 33.12 Jeromine 32.04 Pink Lady 21.45 Super Chief 43.56 | Phloridzin represented 52–67% and 75–83% of the total phenolics measured by the Folin-Ciocalteu assay and HPLC method, respectively. Chewing gum could be a suitable delivering material for phloridzin uptake originated from apple seeds. 5 min was enough for the dissolution of almost all added phloridzin (88.43–96%). | [213] |
| Hot water extraction (HWE): Boiling water with 1% acetic acid at S/L=1/60 g/mL during 10 min. | Apple pomace obtained after processing of a | Total phenols (µg GAE of extract/mL) HWE: 10.7 pHWE: 149 | pHWE was mainly composed of flavonols: quercetin-3-O-galactoside (27%), quercetin-3-O- rhamnoside (23%), quercetin- 3-O-arabinofuranoside (13%), and | DPPH (EC50, µg of extract/mL) HWE: 1339 pHWE: 82.4 ABTS (EC50, µg of extract/mL) | HWE represented 29% of dry apple pomace and presented 11 g/kg of polyphenols. 3.26 g GAE of polyphenols per kg of apple pomace were extracted. | |
| Fractionation processes of HWE extracts: ultrafiltration to obtain LMWM (low molecular weight material) and HMWM (high molecular weight material); SPE to obtain polyphenol-isolated fraction (pHWE) and non-retained material (NrFr). | mixture of apples (Royal Gala variety), employing milling, enzymatic digestion and pressing processes, afterwards it was freeze-dried. | Ultrafiltration: 11% of the polyphenols from the HWE remained in the HMWM. This fraction accounted for 6.9% of the apple pomace. SPE: The hydrophobic fraction pHWE corresponded to 1.6% of the dry apple pomace and accounted for 63% of those in the HWE. | the dihydrochalcone phloretin-2-O-glucoside (14%). | HWE: 532 pHWE: 35.2 | When apple pomace HWE were added to yogurt formulations, a final product with improved fiber content and antioxidant properties was achieved in comparison to control sample (plain yogurt). | [214] |
| Ultrasound Assisted Extraction (UAE): Extraction with aqueous acetone (70/30 vol) for 5 min in an ultrasonic bath at 20°C. | Apple seeds oil and defatted seed flour from the cider- making industry | In defatted seeds flour: Total phenols 2.7-6.7 mg GAE/g defatted matter Condensed tannins 2.4-4.0 mg cyanidin/g defatted matter Hydrolysable tannins 34.5-47.2 mg GAE/g defatted matter | In apple seeds oil: DPPH IC50 = 4.8-5.8 mg of oil/mL | Apple seeds oil exhibited an significant antioxidant activity due to high levels of tocopherols (1280 ± 104.8 mg/kg oil) with β-tocopherol (794.5 ± 62.2 mg/kg oil) being most abundant, followed by α-tocopherol (439.2 ± 34.5 mg/kg oil). Defatted seeds flour contained considerable amounts of phenolic compounds. | [215] | |
| Microwave-assisted extraction (MAE): MAE parameters: solvent type: 70% acetone (Ac), 60% ethanol (Et); microwave power: 100–900W; solvent volume to sample ratio: 4–12 mL/g dry pomace; extraction time: 30–180 s. | Freeze-dried apple pomace of Red Delicious (RD) and Jonathan (J) apple varieties | Total phenols (mg GAE/g DW) under the optimum MAE parameters: RD-Ac: 6.66 RD-Et: 15.81 J-Ac: 5.79 J-Et: 7.65 | Phenolic compounds (µg/g dry pomace) Phloridzin RD-Ac: 82.59 RD-Et: 25.25 J-Ac: 102.9 J-Et: 79.80 Gallic acid: RD-Ac: nd RD-Et: 189.09 J-Ac: nd J-Et: 23.08 Caffeic acid: RD-Ac: 10.00 RD-Et: 27.63 J-Ac: 9.77 J-Et: 34.67 Chlorogenic acid: RD-Ac: nd RD-Et: nd J-Ac: nd J-Et: 22.56 Quercetin: RD-Ac: 46.33 RD-Et: 59.20 J-Ac: 115.15 J-Et: 211.34 | DPPH (%) 60% ethanol: 77.1 70% acetone: 93.7 | The optimum conditions for MAE: 735 W power and 149 s extraction time with 10.3 mL of ethanol per gram dry sample. Higher total phenols and DPPH radical scavenging activity was found in Red Delicious apple pomace extracts. Extraction solvent was not found as significant extraction parameter. | [216] |
| Conventional extraction (CE): agitation (1000 rpm) of samples (2.5 g) in water (50 mL) at room temperature. Ultrasound-assisted extraction (UAE): 10 g of sample in 200 mL of water ultrasonic (US) probe diameter: 1.2 cm, height: 13.7 cm at a depth of ca. 20 mm. US power: 7.8 or 49.5 W The aliquots were sampled every 2, 5, 10, 20 and 30 min and stored until further analysis. Microwave-assisted extraction (MAE): 10 g of sample in 200 mL of water. Power: 400, 600, 1000 W (which corresponds to the actual power of 302, 384, and 435 W) for 2 or 4 irradiation cycles, each consisted of 1 or 1.5 min. High speed homogenization (HH)+MAE: Samples were pre-treated with a high-speed homogenizer prior to MAE. Homogenization at 22,000 rpm for 1 min + MAE: 1000 W for 4 cycles of 1.5 min each. Ultrasound-assisted enzymatic extraction (UAEE): the same as UAE + the pectinase solution | Apple pomace consisted of stalk, skin and the fruit kernel were mixed thoroughly and ground into powder. | Total phenols (mg GAE/g raw sample) CE: 2.62-2.68 UAE: 0.7-2.11 MAE: 2.23-2.81 HH: 2.32-2.44 HH+MAE: 2.65-2.78 UAEE: 2.50-4.62 | LC-MS analysis of fractions obtained from UAEE revealed the presence of 26 phenolic compounds. | The DPPH scavenging ability of the extracts ranged from 27.1 to 54.6 ± 6.9 mg trolox/L, depending on the extraction parameters. | In most cases, extraction time and the particle size of the sample had significant effect on the extraction yield and longer extraction times lead to increased TPC in the extract. UAEE found to be superior techniques to extract TPC from apple pomace due to the synergistic effects of US and enzymes. The antioxidant activity decreased with the extraction time and enzyme dosage. | [217] |
| Mild intensity pulsed electric field (PEF) PEF treatment: 1, 2 and 3 kV/cm 500, 875 and 1250 μs The specific energy intake: 0.44-17.0 kJ/kg Extraction: Fluor to water ratio (FWR): 5, 8.75, 12.5% (w/v) Centrifugation with methanol. Free phenolic acid extraction: shaking with 80% aqueous methanol, centrifugation, evaporation and | Fermented apple pomace powder (12.5% w/v). | Total phenols 402.7 mg GAE/100g DW (at 12.5% FWR) | Phenolic compounds (μg/g DW) Control samples: Protocatechuic acid: 392.6 Chlorogenic acid: 72.5 Caffeic acid: 130.8 p-Coumaric acid: 24.5 Ferulic acid: 64.4 Salicylic acid: 131.9 PEF treated samples: Protocatechuic acid: 98.9 Caffeic acid: 141.1 p-Coumaric acid: 34.3 Ferulic acid: 70.1 Salicylic acid: 170.2 | DPPH 799.3 μmol TE/100g DW (at 12.5% FWR) | Total phenols and AA were significantly (p < 0.05) influenced by the FWR and its interaction with the electric filed intensity and time. With the increase in electric filed intensity from 1 kV/cm to 3 kV/cm, a significant (p < 0.05) increase of 4.8% TPC and 4.4% AA was observed. A significant (p < 0.05) decrease of 3.8% and 2.6% was observed in total phenols and AA, respectively with the increase in treatment time from 500 μs to 875 μs followed by no significant (p > 0.05) change in both total phenols and AA. The optimum condition of PEF treatment was found as 12.5% (w/v) FWR, 2 kV/cm electric field intensity and 500 μs treatment time with a specific energy input of 3.0 kJ/kg. Obtained results indicated that the PEF treatment could be useful tool for the processing of food with enhanced levels of phenolic antioxidants. | [218] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fidelis, M.; de Moura, C.; Kabbas Junior, T.; Pap, N.; Mattila, P.; Mäkinen, S.; Putnik, P.; Bursać Kovačević, D.; Tian, Y.; Yang, B.; et al. Fruit Seeds as Sources of Bioactive Compounds: Sustainable Production of High Value-Added Ingredients from By-Products within Circular Economy. Molecules 2019, 24, 3854. https://doi.org/10.3390/molecules24213854
Fidelis M, de Moura C, Kabbas Junior T, Pap N, Mattila P, Mäkinen S, Putnik P, Bursać Kovačević D, Tian Y, Yang B, et al. Fruit Seeds as Sources of Bioactive Compounds: Sustainable Production of High Value-Added Ingredients from By-Products within Circular Economy. Molecules. 2019; 24(21):3854. https://doi.org/10.3390/molecules24213854
Chicago/Turabian StyleFidelis, Marina, Cristiane de Moura, Tufy Kabbas Junior, Nora Pap, Pirjo Mattila, Sari Mäkinen, Predrag Putnik, Danijela Bursać Kovačević, Ye Tian, Baoru Yang, and et al. 2019. "Fruit Seeds as Sources of Bioactive Compounds: Sustainable Production of High Value-Added Ingredients from By-Products within Circular Economy" Molecules 24, no. 21: 3854. https://doi.org/10.3390/molecules24213854
APA StyleFidelis, M., de Moura, C., Kabbas Junior, T., Pap, N., Mattila, P., Mäkinen, S., Putnik, P., Bursać Kovačević, D., Tian, Y., Yang, B., & Granato, D. (2019). Fruit Seeds as Sources of Bioactive Compounds: Sustainable Production of High Value-Added Ingredients from By-Products within Circular Economy. Molecules, 24(21), 3854. https://doi.org/10.3390/molecules24213854