Antiviral, Antimicrobial and Antibiofilm Activity of Selenoesters and Selenoanhydrides
Abstract
:1. Introduction
2. Results
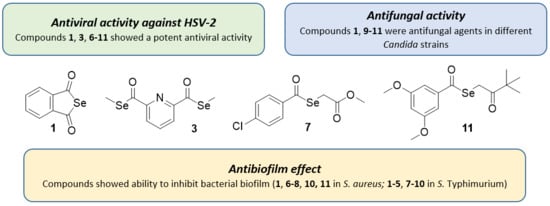
2.1. Antiviral Activity against HSV
2.2. Antifungal Activity
2.3. Antibacterial Activity against Anaerobes
2.4. Antibiofilm Activity against S. Aureus and S. Typhimurium
2.5. Antibiofilm Activity against S. Aureus and S. Typhimurium in Combination with Antibiotics
3. Discussion
3.1. Antiviral Activity against HSV
3.2. Antifungal Activity
3.3. Antibacterial Activity against Anaerobes
3.4. Antibiofilm Activity against S. aureus and S. Typhimurium
3.5. Antibiofilm Activity against S. aureus and S. Typhimurium in Combination with Antibiotics
4. Materials and Methods
4.1. Chemistry
4.2. Strains and Cell Lines Used
4.2.1. Viral Strains
4.2.2. Cell Lines
4.2.3. Fungal Strains
4.2.4. Bacterial Strains
4.3. Assay for Anti-HSV-2 Activity
4.4. Antifungal Activity against Pathogenic Yeasts
4.5. Antibacterial Activity against Anaerobic Bacteria
4.6. Measuring Biofilm Formation Using Crystal Violet
4.7. Enhancement of the Activity of Antibiotics against Biofilm Formation
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, Z.; Xu, X.; Leng, X.; He, M.; Wang, J.; Cheng, S.; Wu, H. Roles of reactive oxygen species in cell signaling pathways and immune responses to viral infections. Arch. Virol. 2017, 162, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Camini, F.C.; da Silva Caetano, C.C.; Almeida, L.T.; de Brito Magalhães, C.L. Implications of oxidative stress on viral pathogenesis. Arch. Virol. 2017, 162, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.; Yao, H.; Nan, Y.; Qiao, L. Role of oxidative stress in hepatitis C virus induced hepatocellular carcinoma. Curr. Cancer Drug Targets 2017, 17, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Reshi, M.L.; Su, Y.C.; Hong, J.R. RNA viruses: ROS-mediated cell death. Int. J. Cell. Biol. 2014, 2014, 467452. [Google Scholar] [CrossRef] [PubMed]
- Nencioni, L.; Sgarbanti, R.; Amatore, D.; Checconi, P.; Celestino, I.; Limongi, D.; Anticoli, S.; Palamara, A.T.; Garaci, E. Intracellular redox signaling as therapeutic target for novel antiviral strategy. Curr. Pharm. Des. 2011, 17, 3898–3904. [Google Scholar] [CrossRef]
- Misra, S.; Boylan, M.; Selvam, A.; Spallholz, J.E.; Björnstedt, M. Redox-active selenium compounds - from toxicity and cell death to cancer treatment. Nutrients 2015, 7, 3536–3556. [Google Scholar] [CrossRef]
- Wrobel, J.K.; Power, R.; Toborek, M. Biological activity of selenium: Revisited. IUBMB Life 2016, 68, 97–105. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Mukherjee, S.; Weiner, W.S.; Schroeder, C.E.; Simpson, D.S.; Hanson, A.M.; Sweeney, N.L.; Marvin, R.K.; Ndjomou, J.; Kolli, R.; Isailovic, D.; et al. Ebselen inhibits hepatitis C virus NS3 helicase binding to nucleic acid and prevents viral replication. ACS Chem. Biol. 2014, 9, 2393–2403. [Google Scholar] [CrossRef]
- Sartori, G.; Jardim, N.S.; Marcondes Sari, M.H.; Dobrachinski, F.; Pesarico, A.P.; Rodrigues, L.C.; Cargnelutti, J.; Flores, E.F.; Prigol, M.; Nogueira, C.W. Antiviral Action of Diphenyl Diselenide on Herpes Simplex Virus 2 Infection in Female BALB/c Mice. J. Cell. Biochem. 2016, 117, 1638–1648. [Google Scholar] [CrossRef]
- Pietka-Ottlik, M.; Potaczek, P.; Piasecki, E.; Mlochowski, J. Crucial role of selenium in the virucidal activity of benzisoselenazol-3(2H)-ones and related diselenides. Molecules 2010, 15, 8214–8218. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhi, X.; Sun, G.; Guo, W.; Huang, Y.; Sun, W.; Tian, X.; Zhao, F.; Hu, K. Sodium selenite suppresses hepatitis B virus transcription and replication in human hepatoma cell lines. J. Med. Virol. 2016, 88, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Ramya, S.; Shanmugasundaram, T.; Balagurunathan, R. Biomedical potential of actinobacterially synthesized selenium nanoparticles with special reference to antibiofilm, anti-oxidant, wound healing, cytotoxic and anti-viral activities. J. Trace Elem. Med. Biol. 2015, 32, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, Y.; Gong, G.; Xia, Y.; Wang, C.; Chen, Y.; Hua, L.; Zhong, J.; Tang, Y.; Liu, X.; et al. Restriction of H1N1 influenza virus infection by selenium nanoparticles loaded with ribavirin via resisting caspase-3 apoptotic pathway. Int. J. Nanomed. 2018, 13, 5787–5797. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Guo, M.; Zhao, M.; Xia, Y.; Wang, C.; Xu, T.; Zhu, B. Inhibition of H1N1 influenza virus-induced apoptosis by functionalized selenium nanoparticles with amantadine through ROS-mediated AKT signaling pathways. Int. J. Nanomed. 2018, 13, 2005–2016. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Guo, M.; Xia, Y.; Zhao, M.; Wang, C.; Xu, T.; Chen, T.; Zhu, B. Inhibitory activity of selenium nanoparticles functionalized with oseltamivir on H1N1 influenza virus. Int. J. Nanomed. 2017, 12, 5733–5743. [Google Scholar] [CrossRef]
- Sahu, P.K.; Umme, T.; Yu, J.; Nayak, A.; Kim, G.; Noh, M.; Lee, J.Y.; Kim, D.D.; Jeong, L.S. Selenoacyclovir and selenoganciclovir: Discovery of a new template for antiviral agents. J. Med. Chem. 2015, 58, 8734–8738. [Google Scholar] [CrossRef]
- Qiao, J.; Zhao, C.; Liu, J.; Du, Y. Design and synthesis of selenazole-substituted ritonavir analogs. Bioorg. Med. Chem. Lett. 2018, 28, 2379–2381. [Google Scholar] [CrossRef]
- Lister, J.L.; Horswill, A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front. Cell Infect. Microbiol. 2014, 4, 178. [Google Scholar] [CrossRef]
- Suresh, M.K.; Biswas, R.; Biswas, L. An update on recent developments in the prevention and treatment of Staphylococcus aureus biofilms. Int. J. Med. Microbiol. 2018, 309, 1–12. [Google Scholar] [CrossRef]
- Mohammed, Y.H.E.; Manukumar, H.M.; Rakesh, K.P.; Karthik, C.S.; Mallu, P.; Qin, H.L. Staphylococcus aureus biofilm war and unlocking key’s for antibiofilm drug development. Microb. Pathog. 2018, 123, 147–339. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.O.; Cruz, E.A.; Souza, E.G.F.; Oliveira, T.C.M.; Alvarenga, V.O.; Peña, W.E.L.; Sant’Ana, A.S.; Magnani, M. Predicting adhesion and biofilm formation boundaries on stainless steel surfaces by five Salmonella enterica strains belonging to different serovars as a function of pH, temperature and NaCl concentration. Int. J. Food Microbiol. 2018, 281, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.J.; Kendall, M.M. Salmonella enterica serovar Typhimurium strategies for host adaptation. Front. Microbiol. 2017, 8, 1983. [Google Scholar] [CrossRef] [PubMed]
- Steenackers, H.; Hermans, K.; Vanderleyden, J.; De Keersmaecker, S.C.J. Salmonella biofilms: An overview on occurrence, structure, regulation and eradication. Food Res. Int. 2012, 45, 502–531. [Google Scholar] [CrossRef]
- Lü, H.; Gao, Y.; Shan, H.; Lin, Y. Preparation and antibacterial activity studies of degraded polysaccharide selenide from Enteromorpha prolifera. Carbohydr. Polym. 2014, 107, 98–102. [Google Scholar] [CrossRef]
- Mosolygó, T.; Kincses, A.; Csonka, A.; Tönki, Á.S.; Witek, K.; Sanmartín, C.; Marć, M.A.; Handzlik, J.; Kieć-Kononowicz, K.; Domínguez-Álvarez, E.; et al. Selenocompounds as novel antibacterial agents and bacterial efflux pump inhibitors. Molecules 2019, 24, 1487. [Google Scholar] [CrossRef]
- Witek, K.; Nasim, M.J.; Bischoff, M.; Gaupp, R.; Arsenyan, P.; Vasiljeva, J.; Marć, M.A.; Olejarz, A.; Latacz, G.; Kieć-Kononowicz, K.; et al. Selenazolinium Salts as “Small Molecule Catalysts” with High Potency against ESKAPE Bacterial Pathogens. Molecules 2017, 22, 2174. [Google Scholar] [CrossRef]
- Chitra, S.; Paul, N.; Muthusubramanian, S.; Manisankar, P.; Yogeeswari, P.; Sriram, D. A facile synthesis of carbocycle-fused mono and bis-1,2,3-selenadiazoles and their antimicrobial and antimycobacterial studies. Eur. J. Med. Chem. 2011, 46, 5465–5472. [Google Scholar] [CrossRef]
- Pesarico, A.P.; Sartori, G.; dos Santos, C.F.; Neto, J.S.; Bortolotto, V.; Santos, R.C.; Nogueira, C.W.; Prigol, M. 2,2′-Dithienyl diselenide pro-oxidant activity accounts for antibacterial and antifungal activities. Microbiol. Res. 2013, 168, 563–568. [Google Scholar] [CrossRef]
- Sonkusre, P.; Singh, C.S. Biogenic selenium nanoparticles inhibit Staphylococcus aureus adherence on different surfaces. Colloids Surf. B Biointerfaces 2015, 136, 1051–1057. [Google Scholar] [CrossRef]
- Guisbiers, G.; Wang, Q.; Khachatryan, E.; Mimun, L.C.; Mendoza-Cruz, R.; Larese-Casanova, P.; Webster, T.J.; Nash, K.L. Inhibition of E. coli and S. aureus with selenium nanoparticles synthesized by pulsed laser ablation in deionized water. Int. J. Nanomed. 2016, 11, 3731–3736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, P.A.; Webster, T.J. Selenium nanoparticles inhibit Staphylococcus aureus growth. Int. J. Nanomed. 2011, 6, 1553–1558. [Google Scholar]
- Wang, Q.; Webster, T.J. Short communication: Inhibiting biofilm formation on paper towels through the use of selenium nanoparticles coatings. Int. J. Nanomed. 2013, 8, 407–411. [Google Scholar]
- Cihalova, K.; Chudobova, D.; Michalek, P.; Moulick, A.; Guran, R.; Kopel, P.; Adam, V.; Kizek, R. Staphylococcus aureus and MRSA growth and biofilm formation after treatment with antibiotics and SeNPs. Int. J. Mol. Sci. 2015, 16, 24656–24672. [Google Scholar] [CrossRef] [PubMed]
- Zonaro, E.; Lampis, S.; Turner, R.J.; Qazi, S.J.; Vallini, G. Biogenic selenium and tellurium nanoparticles synthesized by environmental microbial isolates efficaciously inhibit bacterial planktonic cultures and biofilms. Front. Microbiol. 2015, 6, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremonini, E.; Zonaro, E.; Donini, M.; Lampis, S.; Boaretti, M.; Dusi, S.; Melotti, P.; Lleo, M.M.; Vallini, G. Biogenic selenium nanoparticles: Characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts. Microb. Biotechnol. 2016, 9, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Łączkowski, K.Z.; Biernasiuk, A.; Baranowska-Łączkowska, A.; Zielińska, S.; Sałat, K.; Furgała, A.; Misiura, K.; Malm, A. Synthesis, antimicrobial and anticonvulsant screening of small library of tetrahydro-2H-thiopyran-4-yl based thiazoles and selenazoles. J. Enzyme Inhib. Med. Chem. 2016, 31, 24–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kheradmand, E.; Rafii, F.; Yazdi, M.H.; Sepahi, A.A.; Shahverdi, A.R.; Oveisi, M.R. The antimicrobial effects of selenium nanoparticle-enriched probiotics and their fermented broth against Candida albicans. DARU J. Pharm. Sci. 2014, 22, 48. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Álvarez, E.; Plano, D.; Font, M.; Calvo, A.; Prior, C.; Jacob, C.; Palop, J.A.; Sanmartín, C. Synthesis and antiproliferative activity of novel selenoester derivatives. Eur. J. Med. Chem. 2014, 73, 153–166. [Google Scholar] [CrossRef]
- Domínguez-Álvarez, E.; Gajdács, M.; Spengler, G.; Palop, J.A.; Marć, M.A.; Kieć-Kononowicz, K.; Amaral, L.; Molnár, J.; Jacob, C.; Handzlik, J.; et al. Identification of selenocompounds with promising properties to reverse cancer multidrug resistance. Bioorg. Med. Chem. Lett. 2016, 26, 2821–2824. [Google Scholar] [CrossRef] [Green Version]
- Gajdács, M.; Spengler, G.; Sanmartín, C.; Marć, M.A.; Handzlik, J.; Domínguez-Álvarez, E. Selenoesters and selenoanhydrides as novel multidrug resistance reversing agents: A confirmation study in a colon cancer MDR cell line. Bioorg. Med. Chem. Lett. 2017, 27, 797–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csonka, A.; Kincses, A.; Nové, M.; Vadas, Z.; Sanmartín, C.; Domínguez-Álvarez, E.; Spengler, G. Selenoesters and Selenoanhydrides as Novel Agents Against Resistant Breast Cancer. Anticancer Res. 2019, 39, 3777–3783. [Google Scholar] [CrossRef] [PubMed]
- Spengler, G.; Gajdács, M.; Marć, M.A.; Domínguez-Álvarez, E.; Sanmartín, C. Organoselenium compounds as novel adjuvants of chemotherapy drugs-A promising approach to fight cancer drug resistance. Molecules 2019, 24, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brozmanová, J.; Mániková, D.; Vlčková, V.; Chovanec, M. Selenium: A double-edged sword for defense and offence in cancer. Arch. Toxicol. 2010, 84, 919–938. [Google Scholar] [CrossRef] [PubMed]
- Estevam, E.C.; Witek, K.; Faulstich, L.; Nasim, M.J.; Latacz, G.; Domínguez-Álvarez, E.; Kieć-Kononowicz, K.; Demasi, M.; Handzlik, J.; Jacob, C. Aspects of a Distinct Cytotoxicity of Selenium Salts and Organic Selenides in Living Cells with Possible Implications for Drug Design. Molecules 2015, 20, 13894–13912. [Google Scholar] [CrossRef] [Green Version]
- Sanmartín, C.; Plano, D.; Sharma, A.K.; Palop, J.A. Selenium compounds, apoptosis and other types of cell death: An overview for cancer therapy. Int. J. Mol. Sci. 2013, 13, 9649–9672. [Google Scholar] [CrossRef]
- Żesławska, E.; Kincses, A.; Unger, V.; Tóth, V.; Spengler, G.; Nitek, W.; Tejchman, W. Exocyclic Sulfur and Selenoorganic Compounds Towards Their Anticancer Effects: Crystallographic and Biological Studies. Anticancer Res. 2018, 38, 4577–4584. [Google Scholar] [CrossRef]
- Virók, D.P.; Eszik, I.; Mosolygó, T.; Önder, K.; Endrész, V.; Burián, K. A direct quantitative PCR based measurement of herpes simplex virus susceptibility to antiviral drugs and neutralizing antibodies. J. Virol. Methods 2017, 242, 46–52. [Google Scholar] [CrossRef]
- CLSI. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts; CLSI guideline M44-A; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2004. [Google Scholar]
- Rodriguez-Tudela, J.L.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Cuenca-Estrella, M.; Denning, D.; Donnelly, J.P.; Dupont, B.; Fegeler, W.; Moore, C.; et al. Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Clin. Microbiol. Infect. 2003, 9, i–viii. [Google Scholar] [CrossRef] [Green Version]
- González, J.F.; Alberts, H.; Lee, J.; Doolittle, L.; Gunn, J.S. Biofilm Formation Protects Salmonella from the Antibiotic Ciprofloxacin in Vitro and in Vivo in the Mouse Model of chronic Carriage. Sci. Rep. 2018, 8, 222. [Google Scholar] [CrossRef] [Green Version]
- Sóki, J.; Eitel, Z.; Urbán, E.; Nagy, E. Molecular analysis of the carbapenem and metronidazole resistance mechanisms of Bacteroides strains reported in a Europe-wide antibiotic resistance survey. Int. J. Antimicrob. Agents 2013, 41, 122–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matuschek, E.; Brown, D.F.; Kahlmeter, G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaller, M.A.; Messer, S.A.; Jones, R.N.; Castanheira, M. Antifungal susceptibilities of Candida, Cryptococcus neoformans and Aspergillus fumigatus from the Asia and Western Pacific region: Data from the SENTRY antifungal surveillance program (2010-2012). J. Antibiot. 2015, 68, 556–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamier, V.; Ba, L.A.; Jacob, C. Redox active secondary metabolites. Curr. Opin. Chem. Biol. 2011, 15, 149–155. [Google Scholar]
- Sanguinetti, M.; Posteraro, B.; Lass-Flörl, C. Antifungal drug resistance among Candida species: Mechanisms and clinical impact. Mycoses 2015, 58, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Bryan, L.E.; Kowand, S.K.; Van Den Elzen, H.M. Mechanism of aminoglycoside antibiotic resistance in anaerobic bacteria: Clostridium perfringens and Bacteroides fragilis. Antimicrob. Agents Chemother. 1979, 15, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bús, C.; Kúsz, N.; Jakab, G.; Senobar Tahaei, S.A.; Zupkó, I.; Endrész, V.; Bogdanov, A.; Burián, K.; Csupor-Löffler, B.; Hohmann, J.; et al. Phenanthrenes from Juncus compressus Jacq. with promising antiproliferative and anti-HSV-2 activities. Molecules 2018, 23, 2085. [Google Scholar] [CrossRef] [Green Version]
- Rédei, D.; Kúsz, N.; Rafai, T.; Bogdanov, A.; Burián, K.; Csorba, A.; Mándi, A.; Kurtán, T.; Vasas, A.; Hohmann, J. 14-Noreudesmanes and a phenylpropane heterodimer from sea buckthorn berry inhibit Herpes simplex type 2 virus replication. Tetrahedron 2019, 75, 1364–1370. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |





| Compounds | IC50 (µM) |
|---|---|
| 1 | >100 |
| 2 | >100 |
| 3 | >100 |
| 4 | >100 |
| 5 | >100 |
| 6 | >100 |
| 7 | >100 |
| 8 | >100 |
| 9 | 31 |
| 10 | 45 |
| 11 | 39 |
| Cpd. | Conc. on Disk | Inhibition Zone Diameters (in Millimeters) in Different Fungal Strains | |||||
|---|---|---|---|---|---|---|---|
| Cryptococcus diffluens ATCC 32059 | Candida albicans ATCC 10231 | Candida tropicalis ATCC 13803 | Candida krusei ATCC 14243 | Candida glabrata ATCC 36909 | Candida parapsilosis ATCC 22019 | ||
| 1 | 200 µM | - | 14 | 3 | 11 | 2 | - |
| 10 mM | - | >30 | >30 | >30 | 13 | 6 | |
| 6 | 200 µM | - | - | - | - | - | - |
| 10 mM | - | - | - | - | 6 | - | |
| 8 | 200 µM | - | - | - | - | - | - |
| 10 mM | - | - | - | 4 | - | - | |
| 9 | 200 µM | 12 | - | 2 | 19 | 6 | 4 |
| 10 mM | 29 | >30 | 25 | >30 | >30 | >30 | |
| 10 | 200 µM | - | - | - | 14 | - | 2 |
| 10 mM | - | >30 | 16 | >30 | 25 | >30 | |
| 11 | 200 µM | 10 | - | - | - | - | 14 |
| 10 mM | 18 | 19 | - | 12 | 18 | >30 | |
| DMSO | 2 v/v% | - | - | - | - | - | - |
| Cryptococcus diffluens ATCC 32059 | Candida albicans ATCC 10231 | Candida krusei ATCC 14243 | Candida parapsilosis ATCC 22019 | ||||
|---|---|---|---|---|---|---|---|
| 1 | >200 | 1 | 50 | 1 | 100 | 1 | >200 |
| 9 | 100 | 9 | >200 | 9 | 50 | 9 | >200 |
| 10 | >200 | 10 | >200 | 10 | 50 | 10 | >200 |
| 11 | 100 | 11 | >200 | 11 | >200 | 11 | 100 |
| CSP | ND | CSP [51] | ≤0.007–0.110 | CSP [51] | 0.055–0.229 | CSP [51] | 0.110–1.83 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spengler, G.; Kincses, A.; Mosolygó, T.; Marć, M.A.; Nové, M.; Gajdács, M.; Sanmartín, C.; McNeil, H.E.; Blair, J.M.A.; Domínguez-Álvarez, E. Antiviral, Antimicrobial and Antibiofilm Activity of Selenoesters and Selenoanhydrides. Molecules 2019, 24, 4264. https://doi.org/10.3390/molecules24234264
Spengler G, Kincses A, Mosolygó T, Marć MA, Nové M, Gajdács M, Sanmartín C, McNeil HE, Blair JMA, Domínguez-Álvarez E. Antiviral, Antimicrobial and Antibiofilm Activity of Selenoesters and Selenoanhydrides. Molecules. 2019; 24(23):4264. https://doi.org/10.3390/molecules24234264
Chicago/Turabian StyleSpengler, Gabriella, Annamária Kincses, Tímea Mosolygó, Małgorzata Anna Marć, Márta Nové, Márió Gajdács, Carmen Sanmartín, Helen E. McNeil, Jessica M.A. Blair, and Enrique Domínguez-Álvarez. 2019. "Antiviral, Antimicrobial and Antibiofilm Activity of Selenoesters and Selenoanhydrides" Molecules 24, no. 23: 4264. https://doi.org/10.3390/molecules24234264
APA StyleSpengler, G., Kincses, A., Mosolygó, T., Marć, M. A., Nové, M., Gajdács, M., Sanmartín, C., McNeil, H. E., Blair, J. M. A., & Domínguez-Álvarez, E. (2019). Antiviral, Antimicrobial and Antibiofilm Activity of Selenoesters and Selenoanhydrides. Molecules, 24(23), 4264. https://doi.org/10.3390/molecules24234264