Graphene-Like Porous ZnO/Graphene Oxide Nanosheets for High-Performance Acetone Vapor Detection
Abstract
:1. Introduction
2. Results and Discussions
2.1. Materials Characterization
2.2. Sensing Properties
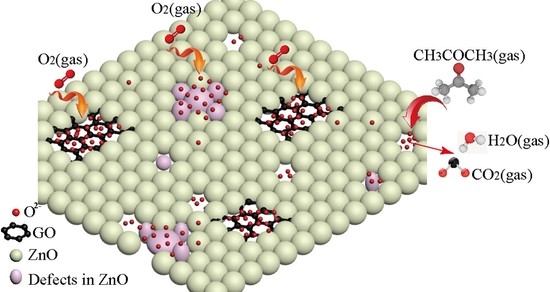
2.3. Sensitive Mechanism
3. Materials and Methods
3.1. Materials
3.2. GO Templated Synthesis of ZnO Nanosheets
3.3. Characterization
3.4. Manufacture and Measurement of Sensors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guo, L.; Kou, X.; Ding, M.; Wang, C.; Dong, L.; Zhang, H.; Feng, C.; Sun, Y.; Gao, Y.; Sun, P.; et al. Reduced graphene oxide/α-Fe2O3 composite nanofibers for application in gas sensors. Sens. Actuators B 2017, 244, 233–242. [Google Scholar] [CrossRef]
- Nasution, T.I.; Nainggolan, I.; Hutagalung, S.D.; Ahmad, K.R.; Ahmad, Z.A. The sensing mechanism and detection of low concentration acetone using chitosan-based sensors. Sens. Actuators B 2013, 177, 522–528. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhu, Z.; Ding, D.; Lu, W.; Xue, Q. Multi-shelled ZnCo2O4 yolk-shell spheres for high-performance acetone gas sensor. Appl. Surf. Sci. 2018, 443, 114–121. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Z.; Liu, S.; Shi, Y.; Dong, Y.; Feng, W. Maize straw-templated hierarchical porous ZnO, Ni with enhanced acetone gas sensing properties. Sens. Actuators B 2017, 243, 1224–1230. [Google Scholar] [CrossRef]
- Singkammo, S.; Wisitsoraat, A.; Sriprachuabwong, C.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Electrolytically exfoliated graphene-loaded flame-made Ni-doped SnO2 composite film for acetone sensing. ACS Appl. Mater. Int. 2015, 7, 3077–3092. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.B.; Bi, H.S.; Li, T.T.; Zhong, X.X.; Chen, X.X.; Fan, A.F.; Wei, D.Z. Low-temperature and highly enhanced NO2 sensing performance of Au-functionalized WO3 microspheres with a hierarchical nanostructure. Appl. Surf. Sci. 2018, 434, 922–931. [Google Scholar] [CrossRef]
- Wang, L.L.; Chen, D.; Jiang, K.; Shen, G.Z. New insights and perspectives into biological materials for flexible electronics. Chem. Soc. Rev. 2017, 46, 6764–6815. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.L.; Zheng, H.X.; Sun, Y.F.; Li, M.Q.; Liu, J.H. Trimethylamine Sensors based on Au modified hierarchical porous single-crystalline ZnO nanosheets. Sensors 2017, 17, 1478. [Google Scholar] [CrossRef]
- Fu, D.; Zhu, C.; Zhang, X.; Li, C.; Chen, Y. Two-dimensional net-like SnO 2/ZnO heteronanostructures for high-performance H 2 S gas sensor. J. Mater. Chem. A 2016, 4, 1390–1398. [Google Scholar] [CrossRef]
- Schütt, F.; Postica, V.; Adelung, R.; Lupan, O. Single and networked ZnO-CNT hybrid tetrapods for selective room-temperature high-performance ammonia sensors. ACS Appl. Mater. Int. 2017, 9, 23107–23118. [Google Scholar] [CrossRef]
- Anasthasiya, A.; Kishore, K.R.; Rai, P.K.; Jeyaprakash, B.G. Highly sensitive graphene oxide functionalized ZnO nanowires for ammonia vapour detection at ambient temperature. Molecules 2018, 23, 3227. [Google Scholar]
- Yu, J.M.; Huang, T.Z.; Jiang, Z.K.; Sun, M.; Tang, C.C. Synthesis and Characterizations of Zinc Oxide on Reduced Graphene Oxide for High Performance Electrocatalytic Reduction of Oxygen. Molecules 2017, 240, 3227. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.R.; Roh, J.G.; Lee, D.D.; Lim, J.O.; Huh, J.S. Sensing behavior of the polypyrrole and polyaniline sensor for several volatile organic compounds. Met. Mater. Int. 2003, 9, 287–291. [Google Scholar] [CrossRef]
- Arockia Jayalatha, K. Room temperature ammonia sensing properties of ZnO thin films grown by spray pyrolysis, Effect of Mg doping. J. Alloy. Compd. 2016, 688, 422–429. [Google Scholar]
- Meng, F.L.; Zheng, H.X.; Chang, Y.L.; Zhao, Y.; Li, M.Q.; Wang, C.; Sun, Y.F.; Liu, J.H. One-step synthesis of Au/SnO2/RGO nanocomposites and their VOC sensing properties. IEEE Trans. Nanotechnol. 2018, 17, 212–219. [Google Scholar] [CrossRef]
- Wang, P.; Wang, D.; Zhang, M.; Zhu, Y.; Xu, Y.; Ma, X.; Wang, X.Y. ZnO nanosheets/graphene oxide nanocomposites for highly effective acetone vapor detection. Sens. Actuators B 2016, 230, 477–484. [Google Scholar] [CrossRef]
- Xue, Z.G.; Cheng, Z.X.; Xu, J.; Xiang, Q.; Wang, X.H.; Xu, J.Q. Controllable evolution of dual defects Zni and Vo associates-rich ZnO nanodishes with (0001) exposed facet and its multiple sensitization effect for ethanol detection. ACS Appl. Mater. Int. 2017, 9, 41559–41567. [Google Scholar] [CrossRef] [PubMed]
- Li, T.M.; He, M.; Zeng, W. Polyhedral Cu2O crystal, Morphology evolution from meshed nanotube to solid and gas-sensing performance. J. Alloy. Compd. 2017, 712, 50–58. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Z.; Song, X.; Yuan, S.; Qiu, Z.; Xu, H.; Cao, B. Improving the triethylamine sensing performance based on debye length, A case study on α-Fe2O3@NiO(CuO) core-shell nanorods sensor working at near room-temperature. Sens. Actuators B 2017, 245, 375–385. [Google Scholar] [CrossRef]
- Paulowicz, I.; Postica, V.; Lupan, O.; Wolff, N.; Shree, S.; Cojocaru, A.; Deng, M.; Kumar Mishra, Y.; Tiginyanu, I.; Kienle, L.; et al. Zinc oxide nanotetrapods with four different arm morphologies for versatile nanosensors. Sens. Actuators B 2018, 262, 425–435. [Google Scholar] [CrossRef]
- Li, Z.J.; Liu, Y.Y.; Guo, D.F.; Guo, J.J.; Su, Y.L. Room-temperature synthesis of CuO/reduced graphene oxide nanohybrids for high-performance NO2 gas sensor. Sens. Actuators B 2018, 271, 306–310. [Google Scholar] [CrossRef]
- Xu, S.H.; Fu, L.; HiepPham, T.S.; Yu, A.M.; Han, F.G.; Chen, L. Preparation of ZnO flower/reduced graphene oxide composite with enhanced photocatalytic performance under sunlight. Ceram. Int. 2015, 41, 4007–4013. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Zhang, H.; Fei, T.; Zhang, T. Enhancing NO2 gas sensing performances at room temperature based on reduced graphene oxide-ZnO nanoparticles hybrids. Sens. Actuators B 2014, 202, 272–278. [Google Scholar] [CrossRef]
- Yoo, R.; Cho, S.; Song, M.J.; Lee, W. Highly sensitive gas sensor based on Al-doped ZnO nanoparticles for detection of dimethyl methyl phosphonate as a chemical warfare agent simulant. Sens. Actuators B 2015, 221, 217–223. [Google Scholar] [CrossRef]
- Xu, J.; Xue, Z.; Qin, N.; Cheng, Z.; Xiang, Q. The crystal facet-dependent gas sensing properties of ZnO nanosheets, Experimental and computational study. Sens. Actuators B 2017, 242, 148–157. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Chen, Z.; Li, H.; Chen, A.; Wang, X.; Yang, J. Enhanced formaldehyde sensing properties of hollow SnO2 nanofibers by graphene oxide. Sens. Actuators B 2017, 250, 533–542. [Google Scholar] [CrossRef]
- Fu, D.; Han, G.; Chang, Y.; Dong, J. The synthesis and properties of ZnO-graphene nanohybrid for photodegradation of organic pollutant in water. Mater. Chem. Phys. 2012, 132, 673–681. [Google Scholar] [CrossRef]
- Hassan, M.M.; Khan, W.; Mishra, P.; Islam, S.S.; Naqvi, A.H. Enhancement in alcohol vapor sensitivity of Cr doped ZnO gas sensor. Mater. Res. Bull. 2017, 93, 391–400. [Google Scholar] [CrossRef]
- Tian, R.; Zhang, Y.Y.; Chen, Z.H.; Duan, H.N.; Xu, B.Y.; Guo, Y.P.; Kang, H.M.; Li, H.; Liu, H.Z. The effect of annealing on a 3D SnO2/graphene foam as an advanced lithium-ion battery anode. Sci. Rep. 2016, 6, 19195. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, T.; Yuan, M.X.; Kang, M.H.; Lu, G.Y.; Wang, R.; Fan, H.T.; He, Y.; Yang, H.B. Growth and selective acetone detection based on ZnO nanorod arrays. Sens. Actuators B 2009, 196, 93–98. [Google Scholar] [CrossRef]
- Tai, H.; Yuan, Z.; Zheng, W.; Ye, Z.; Liu, C.; Du, X. ZnO nanoparticles/reduced graphene oxide bilayer thin films for improved NH3-sensing performances at room temperature. Nanoscale Res. Lett. 2016, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lu, H.; Zhang, J.; Gao, J.; Zhu, G.; Yang, Z.; Yin, F.; Wang, C. Crystal facet-dependent p-type and n-type sensing responses of TiO2 nanocrystals. Sens. Actuators B 2018, 263, 557–567. [Google Scholar] [CrossRef]
- Yu, X.; Song, F.; Zhai, B.; Zheng, C.T.; Wang, Y.D. Electrospun ZnO nanotubes and its gas sensing applications. Phys. E 2013, 52, 92–96. [Google Scholar] [CrossRef]
- Jia, Q.Q.; Ji, H.M.; Zhang, Y.; Chen, Y.L.; Sun, X.H.; Jin, Z.G. Rapid and selective detection of acetone using hierarchical ZnO gas sensor for hazardous odor markers application. J. Hazar. Mater. 2014, 276, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.Y.; Xuan, T.M.; Yin, G.L.; Lu, J.; He, D.N. Controllable synthesis of hierarchical assembled porous ZnO microspheres for acetone gas sensor. Sens. Actuators B 2015, 220, 356–361. [Google Scholar] [CrossRef]
- Koo, W.T.; Choi, S.J.; Jang, J.S.; Kim, I.D. Metal-organic framework templated synthesis of ultrasmall catalyst loaded ZnO/ZnCo2O4 hollow spheres for enhanced gas sensing properties. Sci. Rep. 2017, 7, 45074. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, F.; Xie, N.; Kou, X.; Wang, C.; Sun, Y.; Zhang, T. Ultra-sensitive sensing platform based on Pt-ZnO-In2O3 nanofibers for detection of acetone. Sens. Actuators B 2018, 272, 185–194. [Google Scholar] [CrossRef]
- Chen, H.X.; Yu, H.; Cui, S.; Xu, J.H.; Zhang, Y.; Liu, C.Y. Synthesis of Ce, ZnO nanocomposites, Facile synthesis and fast acetone gas sensing response properties. Phys. B 2017, 51, 636–640. [Google Scholar]
- Liu, C.; Wang, B.Q.; Liu, T.; Sun, P.; Gao, Y.; Liu, F.M.; Lu, G.Y. Facile synthesis and gas sensing properties of the flower-like NiO-decorated ZnO microstructures. Sens. Actuators B 2016, 235, 294–301. [Google Scholar] [CrossRef]
- Bai, S.L.; Guo, J.; Shu, X.; Xiang, X.; Luo, R.X.; Li, D.Q.; Chen, A.F.; Liu, C.C. Surface functionalization of Co3O4 hollow spheres with ZnO nanoparticles for modulating sensing properties of formaldehyde. Sens. Actuators B 2017, 245, 359–368. [Google Scholar] [CrossRef]
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Esfandiar, A.; Irajizad, A.; Akhavan, O.; Ghasemi, S.; Gholami, M.R. Pd-WO3/reduced graphene oxide hierarchical nanostructures as efficient hydrogen gas sensors. Int. J. Hydrog. Energy 2014, 39, 8169–8179. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Zhu, M.; Ju, D.; Xu, H.; Cao, B. High-performance gas sensor based on ZnO nanowires functionalized by Au nanoparticles. Sens. Actuators B 2014, 199, 339–345. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, L.; Wang, B.; Sun, P.; Wang, Q.; Gao, Y.; Lu, G. Acetone gas sensor based on NiO/ZnO hollow spheres, fast response and recovery; and low (ppb) detection limit. J. Colloid Interface Sci. 2017, 495, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhang, H.; Han, D.; Li, J.; Yang, Z.X.; Wang, Q. Preparation of biomorphic porous LaFeO3 by sorghum straw biotemplate method and its acetone sensing properties. Sens. Actuators B 2014, 196, 140–146. [Google Scholar] [CrossRef]
- Wang, D.; Huang, S.; Li, H.; Chen, A.; Wang, P.; Yang, J.; Wang, X.; Yang, J. Ultrathin WO3 nanosheets modified by g-C3N4 for highly efficient acetone vapor detection. Sens. Actuators B 2019, 282, 961–971. [Google Scholar] [CrossRef]
- Wang, D.; Wan, K.; Zhang, M.; Zhang, M.; Li, H.; Wang, P.; Wang, X.; Yang, J. Constructing hierarchical SnO2 nanofiber/nanosheets for efficient formaldehyde detection. Sens. Actuators B 2019, 283, 714–723. [Google Scholar] [CrossRef]
Sample Availability: Samples are available from the authors. |








| Materials | Concentration (ppm) | Response (Ra/Rg) | Recovery Time (s) | Refs |
|---|---|---|---|---|
| ZnO nanotube | 100 | 3.5 | 10 | [34] |
| Mesoporous ZnO | 100 | 33 | 3 | [35] |
| 3D ZnO microsphere | 100 | 22 | 17 | [36] |
| ZnO/ZnCo2O4 | 100 | 7.5 | 36 | [37] |
| Pt-ZnO-In2O3 nanofibers | 100 | 57.1 | 44 | [38] |
| Ce-ZnO nanoparticles | 100 | 20 | 9 | [39] |
| NiO/ZnO microflowers | 100 | 23.5 | 41 | [40] |
| ZnO/GO nanocomposites | 100 | 35.8 | 7 | [17] |
| ZnO/GO NSs | 100 | 94 | 4 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, D.; Tian, L.; Li, H.; Wang, P.; Ou, N.; Wang, X.; Yang, J. Graphene-Like Porous ZnO/Graphene Oxide Nanosheets for High-Performance Acetone Vapor Detection. Molecules 2019, 24, 522. https://doi.org/10.3390/molecules24030522
Wang H, Wang D, Tian L, Li H, Wang P, Ou N, Wang X, Yang J. Graphene-Like Porous ZnO/Graphene Oxide Nanosheets for High-Performance Acetone Vapor Detection. Molecules. 2019; 24(3):522. https://doi.org/10.3390/molecules24030522
Chicago/Turabian StyleWang, Hongwu, Ding Wang, Liang Tian, Huijun Li, Ping Wang, Nanquan Ou, Xianying Wang, and Junhe Yang. 2019. "Graphene-Like Porous ZnO/Graphene Oxide Nanosheets for High-Performance Acetone Vapor Detection" Molecules 24, no. 3: 522. https://doi.org/10.3390/molecules24030522
APA StyleWang, H., Wang, D., Tian, L., Li, H., Wang, P., Ou, N., Wang, X., & Yang, J. (2019). Graphene-Like Porous ZnO/Graphene Oxide Nanosheets for High-Performance Acetone Vapor Detection. Molecules, 24(3), 522. https://doi.org/10.3390/molecules24030522