Hydrolysable Tannins and Biological Activities of Meriania hernandoi and Meriania nobilis (Melastomataceae)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Bio-Guided Phytochemical Study
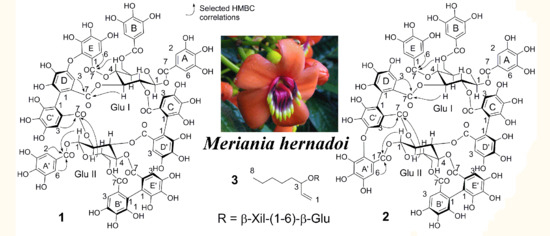
2.2. Characterization of the Compounds
2.3. Bioactivity of Compounds
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Merianin A (1)
3.3.2. Merianin B (2)
3.3.3. Oct-1-en-3-yl β-xylopyranosyl-(1”-6’)-β-glucopyranoside (3)
3.4. Methanolysis and Silylation
3.5. Measurement of DPPH Radical Scavenging Activity
3.6. Measurement of Ferric Reducing Antioxidant Power (FRAP)
3.7. Total Phenolic Content Measured Using the Folin–Ciocalteu Method (TPC)
3.8. Etiolated Wheat Coleoptile Assays
3.9. Standard Target Species Bioassay (STS) and Weeds
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Renner, S.S. Phylogeny and classification of the melastomataceae and memecylaceae. Nord. J. Bot. 1993, 13, 519–540. [Google Scholar] [CrossRef]
- Mimura, M.R.M.; Salatino, A.; Salatino, M.L.F. Distribution of flavonoids and the taxonomy of huberia (melastomataceae). Biochem. Syst. Ecol. 2004, 32, 27–34. [Google Scholar] [CrossRef]
- Mendoza-Cifuentes, H.; Almeda, F.; Alvear, M. Novelties in meriania (melastomataceae: Merianieae) from andean rainforests of colombia. Phytotaxa 2014, 178, 23–32. [Google Scholar] [CrossRef]
- Ocampo Serna, M.D.; Isaza Martínez, H.J. Phenolics and polyphenolics from melastomataceae species. Molecules 2015, 20, 17818–17847. [Google Scholar] [CrossRef] [PubMed]
- Nualkaew, S.; Rattanamanee, K.; Thongpraditchote, S.; Wongkrajang, Y.; Nahrstedt, A. Anti-inflammatory, analgesic and wound healing activities of the leaves of memecylon edule roxb. J. Ethnopharmacol. 2009, 121, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.; Schadow, B.; Quijano, C.E.; Marx, F. Chemical characterization and antioxidant capacity of berries from clidemia rubra (aubl.) mart. (melastomataceae). Food Res. Int. 2011, 44, 2120–2127. [Google Scholar] [CrossRef]
- Nono, R.N.; Barboni, L.; Teponno, R.B.; Quassinti, L.; Bramucci, M.; Vitali, L.A.; Petrelli, D.; Lupidi, G.; Tapondjou, A.L. Antimicrobial, antioxidant, anti-inflammatory activities and phytoconstituents of extracts from the roots of dissotis thollonii cogn. (melastomataceae). S. Afr. J. Bot. 2014, 93, 19–26. [Google Scholar] [CrossRef]
- Vasconcelos, P.C.P.; Kushima, H.; Andreo, M.; Hiruma-Lima, C.A.; Vilegas, W.; Takahira, R.K.; Pellizzon, C.H. Studies of gastric mucosa regeneration and safety promoted by mouriri pusa treatment in acetic acid ulcer model. J. Ethnopharmacol. 2008, 115, 293–301. [Google Scholar] [CrossRef]
- Moleiro, F.C.; Andreo, M.A.; Santos, R.d.C.d.; Moraes, T.d.M.; Rodrigues, C.M.; Carli, C.B.d.A.; Lopes, F.C.M.; Pellizzon, C.H.; Carlos, I.Z.; Bauab, T.M.; et al. Mouririelliptica: Validation of gastroprotective, healing and anti-helicobacter pylori effects. J. Ethnopharmacol 2009, 123, 359–368. [Google Scholar] [CrossRef]
- Durán, A.G.; Gutiérrez, M.T.; Rial, C.; Torres, A.; Varela, R.M.; Valdivia, M.M.; Molinillo, J.M.G.; Skoneczny, D.; Weston, L.A.; Macías, F.A. Bioactivity and quantitative analysis of isohexenylnaphthazarins in root periderm of two Echium spp.: E. plantagineum and E. gaditanum. Phytochemistry 2017, 141, 162–170. [Google Scholar] [CrossRef]
- Macías, F.A.; Castellano, D.; Molinillo, J.M.G. Search for a standard phytotoxic bioassay for allelochemicals. Selection of standard target species. J. Agric. Food Chem. 2000, 48, 2512–2521. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Ashida, M.; Yazaki, K. Tannis of casuarina and stachyurus species. Part 1. Structures of pendunculagin, casuarictin, strictinin, casuarinin, casuariin, and stachyurin. J. Chem. Soc. 1983, 1765–1772. [Google Scholar] [CrossRef]
- Yoshida, T.; Ohbayashi, H.; Ishihara, K.; Ohwashi, W.; Haba, K.; Okano, Y.; Okuda, T.; Shingu, T. Tannins and related polyphenols of melastomataceous plants: I: Hydrolyzable tannins from tibouchina semidecandra cogn. Chem. Pharm. Bull. 1991, 39, 2233–2240. [Google Scholar] [CrossRef]
- Saijo, R.; Nonaka, G.-I.; Nishioka, I. Tannins and related compounds. Lxxxiv.: Isolation and characterization of five new hydrolyzable tannins from the bark of mallotus japonicus. Chem. Pharm. Bull. 1989, 37, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Yoshimoto, M.; Yahara, S.; Okuno, S.; Ishiguro, K.; Yamakawa, O. Identification and characterization of foliar polyphenolic composition in sweetpotato (Ipomoea batatas L.) genotypes. J. Agric. Food Chem. 2002, 50, 3718–3722. [Google Scholar] [CrossRef]
- Zhong, X.-N.; Otsuka, H.; Ide, T.; Hirata, E.; Takushi, A.; Takeda, Y. Three flavonol glycosides from leaves of myrsine seguinii. Phytochemistry 1997, 46, 943–946. [Google Scholar] [CrossRef]
- Napolitano, J.G.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Complete 1h nmr spectral analysis of ten chemical markers of ginkgo biloba. Magn. Reson. chem. 2012, 50, 569–575. [Google Scholar] [CrossRef]
- Hooper, W.; Mahadevan, A. Degradation of catechin by bradyrhizobium japonicum. Biodegradation 1997, 8, 159–165. [Google Scholar] [CrossRef]
- Matsubara, Y.; Sawabe, A.; Iba, H.; Iizuka, Y. Structure of terpenoid glycosides in the leaf of hinoki (chamaecyparies obtusa sieb, et zucc.). Agric. Biol. Chem. 1990, 54, 555–556. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T. Hydrolyzable tannins and related polyphenols. In Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Berlinck, R.G.S., Hatano, T., Okuda, T., Yoshida, T., Herz, W., Kirby, G.W., Moore, R.E., Steglich, W., Tamm, C., Eds.; Springer: Vienna, Austria, 1995; pp. 1–117. [Google Scholar]
- Isaza, J.H.; Ito, H.; Yoshida, T. Oligomeric hydrolyzable tannins from monochaetum multiflorum. Phytochemistry 2004, 65, 359–367. [Google Scholar] [CrossRef]
- Yoshida, T.; Jin, Z.-X.; Okuda, T. Heterophylliins a, b, c, d and e, ellagitannin monomers and dimers from corylus heterophylla fisch. Chem. Pharm. Bull. 1991, 39, 49–54. [Google Scholar] [CrossRef]
- Yoshida, T.; Ohwashi, W.; Haba, K.; Ohbayashi, H.; Ishihara, K.; Okano, Y.; Shingu, T.; Okuda, T. Tannins and related polyphenols of melastomataceous plants. Ii. Nobotanins b, c and e, hydrolyzable tannin dimer and trimers from tibouchina semidecandra cogn. Chem. Pharm. Bull. 1991, 39, 2264–2270. [Google Scholar] [CrossRef]
- Yoshida, T.; Chen, L.; Shingu, T.; Okuda, T. Tannins and related polyphenols of euphorbiaceous plants. Iv.: Euphorbins a and b, novel dimeric dehydroellagitannins from Euphorbia hirta L. Chem. Pharm. Bull. 1988, 36, 2940–2949. [Google Scholar] [CrossRef]
- Ling, S.-K.; Tanaka, T.; Kouno, I. New cyanogenic and alkyl glycoside constituents from phyllagathis rotundifolia. J. Agric. Food Chem. 2002, 65, 131–135. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Marino, T.; Galano, A.; Russo, N. Radical scavenging ability of gallic acid toward oh and ooh radicals. Reaction mechanism and rate constants from the density functional theory. J. Phys. Chem. B 2014, 118, 10380–10389. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, H.; Jiang, Y.; Kusama, K.; Atsumi, T.; Ueha, T.; Toguchi, M.; Iwakura, I.; Satoh, K.; Ito, H.; Hatano, T.; et al. Cytotoxic activity of hydrolyzable tannins against human oral tumor cell lines—A possible mechanism. Phytomedicine 2000, 7, 39–47. [Google Scholar] [CrossRef]
- He, Z.; Lian, W.; Liu, J.; Zheng, R.; Xu, H.; Du, G.; Liu, A. Isolation, structural characterization and neuraminidase inhibitory activities of polyphenolic constituents from flos caryophylli. Phytochem. Lett. 2017, 19, 160–167. [Google Scholar] [CrossRef]
- Cheng, H.-Y.; Lin, C.-C.; Lin, T.-C. Antiherpes simplex virus type 2 activity of casuarinin from the bark of terminalia arjuna linn. Antivir. Res. 2002, 55, 447–455. [Google Scholar] [CrossRef]
- Song, J.H.; Kang, K.S.; Choi, Y.-K. Protective effect of casuarinin against glutamate-induced apoptosis in ht22 cells through inhibition of oxidative stress-mediated mapk phosphorylation. Bioorg. Med. Chem. Lett. 2017, 27, 5109–5113. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.-J.; Bae, Y.-S.; Ju, S.M.; Goh, A.R.; Youn, G.S.; Choi, S.Y.; Park, J. Casuarinin suppresses tarc/ccl17 and mdc/ccl22 production via blockade of nf-κb and stat1 activation in hacat cells. Biochem. Biophys. Res. Commun 2012, 417, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Amakura, Y.; Yoshimura, M. Structural features and biological properties of ellagitannins in some plant families of the order myrtales. Int. J. Mol. Sci. 2010, 11, 79–106. [Google Scholar] [CrossRef] [PubMed]
- Sdiri, S.; Bermejo, A.; Aleza, P.; Navarro, P.; Salvador, A. Phenolic composition, organic acids, sugars, vitamin c and antioxidant activity in the juice of two new triploid late-season mandarins. Food Res. Int. 2012, 49, 462–468. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (frap) as a measure of “antioxidant power”: The frap assay. Anal. Biochem 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Cárdenas, D.M.; Cala, A.; Molinillo, J.M.G.; Macías, F.A. Preparation and phytotoxicity study of lappalone from dehydrocostuslactone. Phytochem. Lett. 2017, 20, 66–72. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1, 2, 4, 7, 8, 10 and 11 are available from the authors. |






| FRS50 (μM) a | FRAP (mg Fe2+/100 g DE) | TPC (mg AG/g DE) b | FRAP (µM Fe2+) | |
|---|---|---|---|---|
| Mn 2.1 | 0.28 ± 0.01 | 12 ± 0.1 | ||
| Mn 2.2 | 2.01 ± 0.08 | 26 ± 2 | ||
| Mn 2.3 | 0.57 ± 0.03 | 24 ± 1 | ||
| Mh 2.1 | 53.2 ± 1.5 | 224 ± 7 | ||
| Mh 2.2 | 64.5 ± 1.5 | 240 ± 9 | ||
| Mh 2.3 | 7.45 ± 0.20 | 120 ± 3 | ||
| Quercetin | 14 ± 1 | 60.8 ± 1.3 | 337 ± 1 | |
| 1 | 2.0 ± 0.3 | 366 ± 5 | ||
| 2 | 1.9 ± 0.1 | 364 ± 5 | ||
| 3 | 19 ± 2 | 168 ± 1 | ||
| 4 | 3.3 ± 0.6 | 274 ± 1 | ||
| 5 | 7.5 ± 0.1 | 268 ± 1 | ||
| 6 | c | c | ||
| 7 | 4.1 ± 0.1 | 352 ± 6 | ||
| 8 | c | c | ||
| 9 | 19 ± 1 | 252 ± 2 | ||
| 11 | 19 ± 1 | 263 ± 1 | ||
| 12 | 48 ± 1 | 166 ± 3 | ||
| 13 | 19 ± 1 | 283 ± 3 | ||
| 14 | 473 ± 3 | 44 ± 1 |
| 1 a | 2 b | Tellimagrandin II [22] | 1,2,3,4,6-pentagalloyl-O-β-glucose [24] | |||||
|---|---|---|---|---|---|---|---|---|
| Glc I | 1H | 13C | 1H | 13C | 1H | 13C | 1H | 13C |
| 1 | 6.10 d (J = 8.5) | 92.7 | 6.09 d (J = 8.4) | 91.2 | 6.39 d (J = 9.5) | 93.4 | ||
| 2 | 5.23 dd (J = 8.5, 10) | 76.1 | 5.22 dd (J = 8.4, 10) | 76.2 | 5.66 t (J = 9.5) | 71.9 | ||
| 3 | 5.54 ft (J = 9.6) | 78.4 | 5.53 dd (J = 8.4, 10.7) | 77 | 6.06 t (J = 9.5) | 73.5 | ||
| 4 | 5.92 ft (J = 9.6) | 67.5 | 5.89 ft (J = 8.9) | 68.5 | 5.70 t (J = 9.5) | 69.5 | ||
| 5 | 3.44 d (J = 9.6) | 74.3 | 3.43 d (J = 8.9) | 74.7 | 4.60 m | 74.1 | ||
| 6 | 3.79 dd (J = 2.7, 13.5) | 64.0 | 3.77 d (J = 11) | 62.6 | 4.60 m | 62.9 | ||
| 4.77 d (J = 13.5) | 4.76 d (J = 11) | 4.45 dd (J = 3, 12) | ||||||
| Glc II | ||||||||
| 1 | 6.24 d (J = 8.7) | 92.7 | 6.24 d (J = 8.4) | 91.3 | 6.20 d (J = 8.0) | 93.8 | ||
| 2 | 5.09 ft (J = 8.7) | 77.0 | 5.08 ft (J = 9.8) | 75.5 | 5.58 dd (J = 8.0, 9.5) | 71.8 | ||
| 3 | 5.73 dd (J = 8.7, 10) | 77.6 | 5.73 ft (J = 9.8) | 76.2 | 5.83 t (J = 9.5) | 73.3 | ||
| 4 | 5.16 ft (J = 10) | 69.9 | 5.15 ft (J = 9.8) | 68.5 | 5.20 t (J = 9.5) | 70.8 | ||
| 5 | 4.58 dd (J = 6.9, 10) | 73.8 | 4.57 dd (J = 6.4, 13.5) | 72.4 | 4.54 dd (J = 6.0, 9.5) | 73.1 | ||
| 6 | 3.93 dd (J = 2, 13.6) | 63.9 | 3.92 d (J = 11.6) | 62.4 | 5.36 dd (J = 6.0, 13) | 63.1 | ||
| 5.38 dd (J = 6.9, 13.6) | 5.37 dd (J = 6.4, 11.6) | 3.87 d (J = 13) | ||||||
| 1 a | 2 b | ||||
|---|---|---|---|---|---|
| Aromatic-H | -COO- | Glucose-H | Aromatic- H b | -COO- | Glucose-H |
| Galloyl | Galloyl | ||||
| 6.96 A-2,6 | 165.7 | 6.24 Glc II (H-1) | 7.22 A-2,6 | 166.6 | 5.37 Glc I (H-6) |
| 7.09 B-2,6 | 165.9 | 6.10 Glc I (H-1) | 7.07 B-2,6 | 164.5 | 6.09 Glc I (H-1) |
| 7.23 A’-2,6 | 168.0 | 5.38 Glc I (H-6) | |||
| 7.11 E-2,6 | 164.3 | 5.89 Glc I (H-4) | |||
| HHDP | HHDP | ||||
| 6.39 C-3 | 170.0 | 5.23 Glc I (H-2) | 6.38 C-3 | 168.6 | 5.22 Glc I (H-2) |
| 6.41 D’-3 | 170.5 | 5.73 Glc II (H-3) | 6.40 D’-3 | 169.1 | 5.73 Glc II (H-3) |
| 6.54 E’-3 | 169.1 | 5.16 Glc II (H-4) | 6.53 E’-3 | 167.7 | 5.15 Glc II (H-4) |
| 6.62 B’-3 | 169.6 | 3.93 Glc II (H-6) | 6.60 B’-3 | 168.2 | 3.92 Glc II (H-6) |
| Valoneoyl | Valoneoyl | ||||
| 6.48 D | 170.7 | 5.54 Glc I (H-3) | 6.47 D | 169.2 | 5.53 Glc I (H-3) |
| 5.91 C’ | 169.5 | 5.09 Glc II (H-2) | 5.89 C’ | 168.1 | 5.08 Glc II (H-2) |
| 7.12 E | 165.7 | 5.92 Glc I (H-4) | |||
| 6.95 A’ | 164.3 | 6.24 Glc II (H-1) |
| 1H | 13C | 1H | 13C | |
|---|---|---|---|---|
| Glucose | Xylose | |||
| 1 | 4.35 d (J = 7.8) | 102.5 | 4.34 d (J = 7.6) | 102.5 |
| 2 | 3.21 dd (J = 7.8, 9.1) | 73.8 | 3.21 dd (J = 7.6, 9.1) | 74.3 |
| 3 | 3.41 dd (J = 6.5, 9.1) | 77.1 | 3.36 ft (J = 8.8) | 75.8 |
| 4 | 3.39 dd (J = 6.5, 8.1) | 76.6 | 3.49 dd (J = 4.8, 8.5) | 70.2 |
| 5 | 3.43 dd (J = 8.1 10.4) | 70.1 | 3.70 dd (J = 4.8, 11.5) | 68.7 |
| 3.98 dd (J = 2.1, 11.5) | ||||
| 6 | 3.18 dd (J = 10.1, 11.4) | 65.9 | ||
| 3.81 dd (J = 5.3, 11.4) | ||||
| Aglicone | ||||
| 1a | 5.18 dd (J = 3.6, 17.3) | 116.1 | ||
| 1b | 5.05 dd (J = 3.6, 5.5) | |||
| 2 | 5.82 ddd (J = 7.1, 10.5, 17.3) | 140.2 | ||
| 3 | 4.09 q (J = 5.5, 8.7) | 82.1 | ||
| 4a | 1.45 d (J = 8.7) | 34.9 | ||
| 4b | 1.60 dd (J = 10, 13.6) | |||
| 5 | 1.22 d (J = 11, 13.6) | 32.2 | ||
| 6 | 1.22 d (J = 11) | 24.9 | ||
| 7 | 1.30 ft (J = 11, 7.0) | 22.9 | ||
| 8 | 0.8 ft (J = 7.2) | 14.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valverde Malaver, C.L.; Colmenares Dulcey, A.J.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Macías, F.A.; Isaza Martínez, J.H. Hydrolysable Tannins and Biological Activities of Meriania hernandoi and Meriania nobilis (Melastomataceae). Molecules 2019, 24, 746. https://doi.org/10.3390/molecules24040746
Valverde Malaver CL, Colmenares Dulcey AJ, Rial C, Varela RM, Molinillo JMG, Macías FA, Isaza Martínez JH. Hydrolysable Tannins and Biological Activities of Meriania hernandoi and Meriania nobilis (Melastomataceae). Molecules. 2019; 24(4):746. https://doi.org/10.3390/molecules24040746
Chicago/Turabian StyleValverde Malaver, Claudia Lorena, Ana Julia Colmenares Dulcey, Carlos Rial, Rosa M. Varela, José M. G. Molinillo, Francisco A. Macías, and José Hipólito Isaza Martínez. 2019. "Hydrolysable Tannins and Biological Activities of Meriania hernandoi and Meriania nobilis (Melastomataceae)" Molecules 24, no. 4: 746. https://doi.org/10.3390/molecules24040746
APA StyleValverde Malaver, C. L., Colmenares Dulcey, A. J., Rial, C., Varela, R. M., Molinillo, J. M. G., Macías, F. A., & Isaza Martínez, J. H. (2019). Hydrolysable Tannins and Biological Activities of Meriania hernandoi and Meriania nobilis (Melastomataceae). Molecules, 24(4), 746. https://doi.org/10.3390/molecules24040746