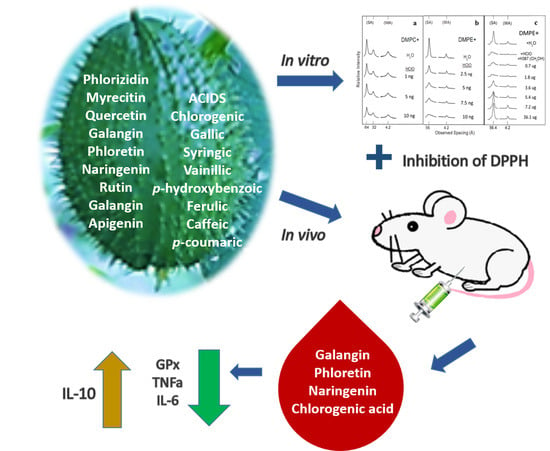
Phytochemical Analysis and Antioxidant and Anti-Inflammatory Capacity of the Extracts of Fruits of the Sechium Hybrid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Analysis of the Extract of Fruits of the Hybrid of Sechium H387 07
2.2. In Vitro Antioxidant Activity of Sechium H387 07 Fruit Extract
2.3. Protection of Model Membrane Structure by Sechium H387 07 Hybrid Extract
2.4. Phenolic Compounds from Sechium H387 07 Fruit Extract that Enter the Bloodstream
2.5. Reduction in Inflammatory Cytokines in Healthy Mice Treated with Sechium H387 07 Fruit Extract
2.6. Sechium H387 07 Hybrid Extract Reduces Oxidative Stress Damage in Mice
3. Materials and Methods
3.1. Material and Chemicals
3.2. Extraction
3.3. Identification of the Total Phenolic Content (TPC)
3.4. Identification of Compounds by High-Performance Liquid Chromatography (HPLC)
3.5. In Vitro Antioxidant Activity
3.6. In Vivo Antioxidant Activity
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosado-Pérez, J.; Aguiñiga-Sánchez, I.; Arista-Ugalde, T.L.; Santiago-Osorio, E.; Mendoza-Nuñez, V.M. The biological significance of oxidative stress, effects of fruits as natural edible antioxidants. Curr. Pharm. Des. 2018, 24, 4807–4824. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- World Health Assembly, 66. Follow-Up to the Political Declaration of the High-Level Meeting of the General Assembly on the Prevention and Control of Non-Communicable Diseases. 2013. Available online: https://apps.who.int/iris/handle/10665/150161 (accessed on 12 March 2020).
- Bjørklund, G.; Chirumbolo, S. Role of oxidative stress and antioxidants in daily nutrition and human health. Nutrition 2017, 33, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Aguiñiga-Sánchez, I.; Cadena-Íñiguez, J.; Santiago-Osorio, E.; Gómez-García, G.; Mendoza-Núñez, V.M.; Rosado-Pérez, J.; Ruíz-Ramos, M.; Cisneros-Solano, V.M.; Ledesma-Martínez, E.; Delgado-Bordonave, A.J.; et al. Chemical analyses and in vitro and in vivo toxicity of fruit methanol extract of Sechium edule var. nigrum spinosum. Pharm. Biol. 2017, 55, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, T.; De Tommasi, N.; Morelli, I.; Braca, A. Study of flavonoids of Sechium edule (Jacq) Swartz (Cucurbitaceae) different edible organs by liquid chromatography photodiode array mass spectrometry. J. Agric. Food Chem. 2004, 52, 6510–6515. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.A.; Guppy, L.J.; Nelson, M. The antihypertensive effects of the Jamaican Cho-Cho (Sechium edule). West Indian Med. J. 2000, 49, 27–31. [Google Scholar]
- Ordoñez, A.A.L.; Gomez, J.D.; Cudmani, N.M.; Vattuone, M.A.; Isla, M.I. Antimicrobial activity of nine extracts of Sechium edule (Jacq.) Swartz. Microb. Ecol. Health Dis. 2003, 15, 33–39. [Google Scholar] [CrossRef]
- Ordoñez, A.A.L.; Gomez, J.D.; Vattuone, M.A. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J.; Soto-Hernández, M.; Torres-Salas, A.; Aguiñiga, I.; Ruiz-Posadas, L.; Rivera-Martínez, A.R.; Santiago-Osorio, E. The antiproliferative effect of chayote varieties (Sechium edule (Jacq.) Sw.) on tumour cell lines. J. Med. Plant Res. 2013, 7, 455–460. [Google Scholar]
- Mumtaz, S.M.F.; Paul, S.; Bag, A.K. Effect of Sechium edule on chemical induced kidney damage in experimental animals. Bangladesh J. Pharmacol. 2013, 8, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Firdous, S.; Sravanthi, K.A.; Debnath, R.A.; Neeraja, K.A. Protective effect of ethanolic extract and its ethylacetate and n-butanol fractions of Sechium edule fruits against carbon tetrachloride induced hepatic injury in rats. Int. J. Pharm. Pharm. Sc. 2012, 4, 354–359. [Google Scholar]
- Rosado-Pérez, J.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Effect of Sechium edule var. nigrum spinosum (Chayote) on oxidative stress and pro-inflammatory markers in older adults with metabolic syndrome: An exploratory study. Antioxidants 2019, 8, 146. [Google Scholar]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Solano, V.; Cadena-Iñiguez, J.; Avendaño-Arrazate, C.; Arévalo-Galarza, L. Rescatando y aprovechando los recursos fitogeneticos de Mesoamerica. Grupo Interdisciplinario de Investigación en Sechium edule en México, AC. El Chayote 2011, 2, 1–21. [Google Scholar]
- Aguiñiga-Sánchez, I.; Soto-Hernández, M.; Cadena-Iñiguez, J.; Ruíz-Posadas, L.; Cadena-Zamudio, J.; González-Ugarte, A.K.; Weiss, S.B.; Santiago-Osorio, E. Fruit extract from a Sechium edule hybrid induce apoptosis in leukaemic cell lines but not in normal cells. Nutr. Cancer 2015, 67, 250–257. [Google Scholar] [CrossRef]
- Grigalius, I.; Petrikaite, V. Relationship between antioxidant and anticancer activity of trihydroxyflavones. Molecules 2017, 22, 2169. [Google Scholar] [CrossRef] [Green Version]
- Andreicut, A.; Pârvu, A.; Mot, A.C.; Fodor, F.V.; Cătoi, F.A.; Feldrihan, V.; Cecan, M.; Irimie, A. Phytochemical analysis of anti-inflammatory and antioxidant effects of Mahonia aquifolium flower and fruit extracts. Oxid. Med. Cell. Longev. 2018, 2018, 1–12. [Google Scholar]
- Bacanli, M.; Başaran, A.A.; Başaran, N. Galangin as a plant phenolic and usage in health and disease. In Polyphenols: Prevention and Treatment of Human Disease; Academic Press: Cambridge, MA, USA, 2018; pp. 433–438. [Google Scholar]
- Hong, Y.; Yin, Y.; Tan, Y.; Hong, K.; Zhou, H. The Flavanone, Naringenin, modifies antioxidant and Steroidogenic enzyme activity in a rat model of Letrozole-induced polycystic ovary syndrome. Med. Sci. Monit. 2019, 25, 395–401. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Boon, J.; Smith, B. Chemical control of phospholipid distribution across bilayer membranes. Med. Res. Rev. 2002, 22, 251–281. [Google Scholar] [CrossRef]
- Zavodnik, I.B.; Lapshina, E.A.; Zavodnik, L.B.; Bartosz, G.; Soszynski, M.; Bryszewska, M. Hypochlorous acid damages erythrocyte membrane proteins and alters lipid bilayer structure and fluidity. Free Radic. Biol. Med. 2001, 30, 363–369. [Google Scholar] [CrossRef]
- Suwalsky, M.; Colina, J.; Gallardo, M.J.; Jemiola-Rzeminska, M.; Strzalka, K.; Moreno-Manrique, M.; Sepúlveda, B. Antioxidant capacity of gallic acid in vitro assayed on human erythrocytes. J. Membr. Biol. 2016, 249, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Cassidy, A. A review of the health care potential of bioactive compounds. J. Sci. Food Agric. 2006, 86, 1805–1813. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Bounartzi, M.I.; Georgarakis, M.; Niopas, I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur. J. Clin. Nutr. 2007, 61, 472–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graefe, E.; Wittig, J.; Mueller, S.; Riethling, A.K.; Uehleke, B.; Drewelow, B.; Pforte, H.; Jacobasch, G.; Derendorf, H.; Veit, M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 2001, 41, 492–499. [Google Scholar] [CrossRef]
- Nardini, M.; Cirillo, E.; Natella, F.; Scaccini, C. Absorption of phenolic acids in humans after coffee consumption. J. Agric. Food Chem. 2002, 50, 5735–5741. [Google Scholar] [CrossRef]
- Cartron, E.; Fouret, G.; Carbonneau, M.A.; Lauret, C.; Michel, F.; Monnier, L.; Descomps, B.; Léger, C. Red-wine beneficial long-term effect on lipids but not on antioxidant characteristics in plasma in a study comparing three types of wine--description of two O-methylated derivatives of gallic acid in humans. Free Radic. Res. 2003, 37, 1021–1035. [Google Scholar] [CrossRef]
- Simonetti, P.; Gardana, C.; Pietta, P. Caffeic acid as biomarker of red wine intake. Methods Enzymol. 2001, 335, 122–130. [Google Scholar]
- Bonvehí, J.S.; Coll, F.V. Phenolic composition of propolis from China and from South America. Z. für Nat. C 1994, 49, 712–718. [Google Scholar] [CrossRef]
- Day, A.; Cañada, J.; Díaz, J.; Kroon, P.; Mclauchlan, R.; Faulds, C.; Plumb, W.; Morgan, M.; Williamson, G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef] [Green Version]
- Crespy, V.; Aprikian, O.; Morand, C.; Besson, C.; Manach, C.; Demigné, C.; Rémésy, C. Bioavailability of phloretin and phloridzin in rats. J. Nutr. 2001, 131, 3227–3230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felgines, C.; Texier, O.; Morand, C.; Manach, C.; Scalbert, A.; Régerat, F.; Rémésy, C. Bioavailability of the flavanone naringenin and its glycosides in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G1148–G1154. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I.; Meririnne, E.; Alfthan, G.; Aro, A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J. Nutr. 2001, 131, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Erkekoğlu, P.; Aşçı, A.; Ceyhan, M.; Kizilgun, K.; Schweizer, U.; Atas, C.; Kara, A.; Giray, K. Selenium levels, selenoenzyme activities and oxidant/antioxidant parameters in H1N1-infected children, Turk. J. Pediatr. 2013, 35, 271–282. [Google Scholar]
- Krishnamoorthy, V.; Rather, I.A. Protective effect of Embilica officinalis (amla) on metal-induced lipid peroxidation in human erythrocytes. Pak. J. Med. Sci. 2016, 29, 1023–1026. [Google Scholar]
- Paul, S.; Ghosh, A.K.; Ghosh, D.; Dutta, M.; Mitra, E.; Dey, M.; Bhowmick, D.; Tridid, D.; Firdaus, B.S.; Mishra, S.; et al. Aqueous bark extract of Terminalia arjuna protects against phenylhydrazine induced oxidative damage in goat red blood cell membrane protein, phospholipid asymmetry and structural morphology: A flow cytometric and biochemical analysis. J. Pharm. Res. 2014, 8, 1790–1804. [Google Scholar]
- Borchert, A.; Kalms, J.; Roth, S.R.; Rademacher, M.; Schmidt, A.; Holzhutter, G.H.; Kuhn, H.; Scheerer, P. Crystal structure and functional characterization of selenocysteine-containing glutathione peroxidase 4 suggests an alternative mechanism of peroxide reduction. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2018, 1863, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Afifi, M.S.; Ross, S.A.; Sohly, M.A.; Naeem, Z.E.; Halaweish, F.T. Cucurbitacins of Cucumis prophetarum and Cucumis prophetarum. J. Chem. Ecol. 1999, 25, 847–859. [Google Scholar] [CrossRef]
- Vegliolu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Chang, C.; Yang, M.; Wen, H.; Chern, J. Estimation of total flavonoid content in propolis by two complementary colorimetric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagent. Am. J. Enol. Vitic. 1965, 16, 144–148. [Google Scholar]
- Liu, Y.; Sun, Y.; Laura, T.; Liang, X.; Ye, H.; Zeng, X. Determination of polyphenolic content and antioxidant activity of kudingcha made from Ilex kudingcha, C.J. Tseng. Food Chem. 2009, 112, 35–41. [Google Scholar] [CrossRef]
- Marsh, D. Handbook of Lipid Bilayers, 2nd ed.; CRC Press: London, UK, 2013. [Google Scholar]
- Shih, W.L.; Chang, C.D.; Chen, H.T.; Fan, K.K. Antioxidant activity and leukemia initiation prevention in vitro and in vivo by N-acetyl-L-cysteine. Oncol. Lett. 2018, 16, 2046–2052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguiñiga-Sánchez, I. Efecto Antitumoral in vivo de Sechium P. Browne (Cucurbitaceae). Ph.D. Thesis, Colegio de Postgraduados, Texcoco, Estado de México, México, 2017. [Google Scholar]
Sample Availability: Samples of the compounds flavonoids and phenolic acids listed in Table 1 are available from the authors. |






| H387 07 Hybrid Extract | |||
|---|---|---|---|
| Flavonoids | Phenolic Acids | ||
| (mg/g of Extract) | |||
| Rutin | 1.273 | Gallic acid | 0.056 |
| Phlorizin | 0.0168 | Chlorogenic acid | 4.224 |
| Myricetin | 0.889 | Syringic acid | 0.016 |
| Quercetin | 0.005 | Vanillic acid | 0.087 |
| Naringenin | 3.304 | p-hydroxybenzoic acid | 0.084 |
| Phloretin | 4.616 | Caffeic acid | 0.187 |
| Galangin | 21.940 | Ferulic acid | 0.064 |
| Apigenin | 0.362 | p-coumaric acid | 0.029 |
| Cytokine (pg/mL) | Without Treatment | H387 07 Hybrid Extract (mg/kg) | ||||
|---|---|---|---|---|---|---|
| 0 | 8 | 250 | 500 | 1000 | ||
| TNFα | 255.7 ± 37 | 257.9 ± 26 | 144.8 ± 42 * | 200.4 ± 9 * | 143.1 ± 23 * | 92.46 ± 25 * |
| INFγ | 69.7 ± 6 | 56.6 ± 3 | 49.6 ± 7 | 27.5 ± 8 * | 19.07 ± 6 * | 26.9 ± 3 * |
| IL-6 | 590.0 ± 56 | 512.5 ± 60 | 125.8 ± 47 * | 378.7 ± 20 * | 231 ± 41 * | 237 ± 27 * |
| IL-10 | 342.6 ± 75 | 276.9 ± 82 | 1070 ± 375 * | 2831 ± 168 * | 3870 ± 480 * | 4156 ± 200 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguiñiga-Sánchez, I.; Soto-Hernández, M.; Cadena-Iñiguez, J.; Suwalsky, M.; Colina, J.R.; Castillo, I.; Rosado-Pérez, J.; Mendoza-Núñez, V.M.; Santiago-Osorio, E. Phytochemical Analysis and Antioxidant and Anti-Inflammatory Capacity of the Extracts of Fruits of the Sechium Hybrid. Molecules 2020, 25, 4637. https://doi.org/10.3390/molecules25204637
Aguiñiga-Sánchez I, Soto-Hernández M, Cadena-Iñiguez J, Suwalsky M, Colina JR, Castillo I, Rosado-Pérez J, Mendoza-Núñez VM, Santiago-Osorio E. Phytochemical Analysis and Antioxidant and Anti-Inflammatory Capacity of the Extracts of Fruits of the Sechium Hybrid. Molecules. 2020; 25(20):4637. https://doi.org/10.3390/molecules25204637
Chicago/Turabian StyleAguiñiga-Sánchez, Itzen, Marcos Soto-Hernández, Jorge Cadena-Iñiguez, Mario Suwalsky, José R. Colina, Ivan Castillo, Juana Rosado-Pérez, Víctor M. Mendoza-Núñez, and Edelmiro Santiago-Osorio. 2020. "Phytochemical Analysis and Antioxidant and Anti-Inflammatory Capacity of the Extracts of Fruits of the Sechium Hybrid" Molecules 25, no. 20: 4637. https://doi.org/10.3390/molecules25204637
APA StyleAguiñiga-Sánchez, I., Soto-Hernández, M., Cadena-Iñiguez, J., Suwalsky, M., Colina, J. R., Castillo, I., Rosado-Pérez, J., Mendoza-Núñez, V. M., & Santiago-Osorio, E. (2020). Phytochemical Analysis and Antioxidant and Anti-Inflammatory Capacity of the Extracts of Fruits of the Sechium Hybrid. Molecules, 25(20), 4637. https://doi.org/10.3390/molecules25204637