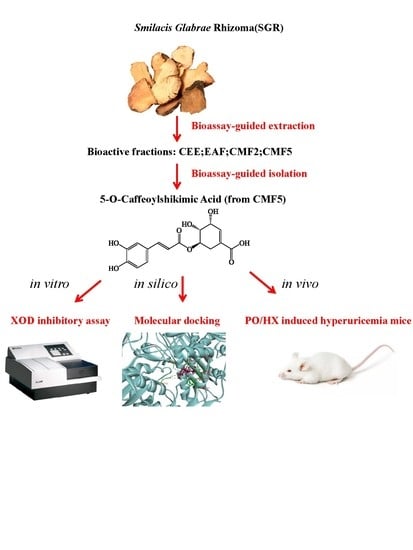
Natural Xanthine Oxidase Inhibitor 5-O-Caffeoylshikimic Acid Ameliorates Kidney Injury Caused by Hyperuricemia in Mice
Abstract
:1. Introduction
2. Results
2.1. Bioassay-Guided Isolation and Identification
2.2. Effects of Compounds on XOD Inhibitory Activity
2.3. Molecular Docking of 5OCSA Acid into XOD
2.4. Effect of 5OCSA on Body Weight and Organ Coefficients in HUA Mice
2.5. Effect of 5OCSA on sUA, Serum XOD and Hepatic XOD
2.6. OCSA Ameliorates Kidney Injury in PO/HX-Induced HUA Mice
2.7. Effect of 5OCSA on Inflammatory Cytokines of Kidney in HUA Mice
3. Discussion
4. Materials and Methods
4.1. Material
4.2. Separation
4.3. XOD Inhibitory Assay and Reversibility Study
4.4. Animals Groupings and Experiment Procedure
4.5. HE Staining and Kidney Histomorphometry
4.6. Measurement of Uric Acid Concentration and XOD Activity
4.7. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, S.; Wang, Y.; Cheng, J.; Huangfu, N.; Zhao, R.; Xu, Z.; Zhang, F.; Zheng, W.; Zhang, D. Hyperuricemia and Cardiovascular Disease. Curr. Pharm. Des. 2019, 25, 700–709. [Google Scholar] [CrossRef]
- Liu, S. Danxi Xinfa and Zhu Danxi’s correlated works. Zhonghua Yi Shi Za Zhi 1995, 25, 111–113. [Google Scholar] [PubMed]
- Shahin, L.; Patel, K.M.; Heydari, M.K.; Kesselman, M.M. Hyperuricemia and Cardiovascular Risk. Cureus 2021, 13, e14855. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yang, N.; Xing, X.; Chang, D.; Li, J.; Deng, J.; Chen, Y.; Hu, C.; Zhang, R.; Lu, X.; et al. Obesity interacts with hyperuricemia on the severity of non-alcoholic fatty liver disease. BMC Gastroenterol. 2021, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Arersa, K.K.; Wondimnew, T.; Welde, M.; Husen, T.M. Prevalence and Determinants of Hyperuricemia in Type 2 Diabetes Mellitus Patients Attending Jimma Medical Center, Southwestern Ethiopia, 2019. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 2059–2067. [Google Scholar] [CrossRef]
- Stewart, D.J.; Langlois, V.; Noone, D. Hyperuricemia and Hypertension: Links and Risks. Integr. Blood Press. Control 2019, 12, 43–62. [Google Scholar] [CrossRef] [Green Version]
- Toda, A.; Ishizaka, Y.; Tani, M.; Yamakado, M. Hyperuricemia is a significant risk factor for the onset of chronic kidney disease. Nephron. Clin. Pract. 2014, 126, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Major, T.J.; Dalbeth, N.; Stahl, E.A.; Merriman, T.R. An update on the genetics of hyperuricaemia and gout. Nat. Rev.. Rheumatol. 2018, 14, 341–353. [Google Scholar] [CrossRef]
- Álvarez-Lario, B.; Alonso-Valdivielso, J.L. Hyperuricemia and gout; the role of diet. Nutr. Hosp. 2014, 29, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, K.; Li, C. Dietary factors and risk of gout and hyperuricemia: A meta-analysis and systematic review. Asia Pac. J. Clin. Nutr. 2018, 27, 1344–1356. [Google Scholar] [PubMed]
- Ben Salem, C.; Slim, R.; Fathallah, N.; Hmouda, H. Drug-induced hyperuricaemia and gout. Rheumatology 2017, 56, 679–688. [Google Scholar] [CrossRef] [Green Version]
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1843–1855. [Google Scholar] [CrossRef]
- Huang, J.; Ma, Z.F.; Zhang, Y.; Wan, Z.; Li, Y.; Zhou, H.; Chu, A.; Lee, Y.Y. Geographical distribution of hyperuricemia in mainland China: A comprehensive systematic review and meta-analysis. Glob. Health Res. Policy 2020, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, A.; Roddy, E.; Doherty, M. Gout—A guide for the general and acute physicians. Clin. Med. 2017, 17, 54–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hainer, B.L.; Matheson, E.; Wilkes, R.T. Diagnosis, treatment, and prevention of gout. Am. Fam. Physician 2014, 90, 831–836. [Google Scholar]
- Mandal, A.K.; Mount, D.B. The molecular physiology of uric acid homeostasis. Annu. Rev. Physiol. 2015, 77, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Bredemeier, M.; Lopes, L.M.; Eisenreich, M.A.; Hickmann, S.; Bongiorno, G.K.; d’Avila, R.; Morsch, A.L.B.; da Silva Stein, F.; Campos, G.G.D. Xanthine oxidase inhibitors for prevention of cardiovascular events: A systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2018, 18, 24. [Google Scholar] [CrossRef]
- Klinenberg, J.R. The effectiveness of allopurinol in the treatment of gout. Arthritis Rheum. 1965, 8, 891–895. [Google Scholar] [CrossRef]
- Bardin, T.; Richette, P. The role of febuxostat in gout. Curr. Opin. Rheumatol. 2019, 31, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.Y.; AlRabiah, H.K.; Al Rashoud, S.S.; Bari, A.; Wani, T.A. Topiramate: Comprehensive profile. Profiles Drug Subst. Excip. Relat. Methodol. 2019, 44, 333–378. [Google Scholar] [PubMed]
- Jordan, A.; Gresser, U. Side Effects and Interactions of the Xanthine Oxidase Inhibitor Febuxostat. Pharmaceuticals 2018, 11, 51. [Google Scholar] [CrossRef] [Green Version]
- Mehmood, A.; Ishaq, M.; Zhao, L.; Safdar, B.; Rehman, A.U.; Munir, M.; Raza, A.; Nadeem, M.; Iqbal, W.; Wang, C. Natural compounds with xanthine oxidase inhibitory activity: A review. Chem. Biol. Drug Des. 2019, 93, 387–418. [Google Scholar] [CrossRef]
- Liang, G.; Nie, Y.; Chang, Y.; Zeng, S.; Liang, C.; Zheng, X.; Xiao, D.; Zhan, S.; Zheng, Q. Protective effects of Rhizoma smilacis glabrae extracts on potassium oxonate- and monosodium urate-induced hyperuricemia and gout in mice. Phytomedicine: Int. J. Phytother. Phytopharm. 2019, 59, 152772. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Shen, R.; Lin, D.; Chen, Y.; Ye, H. Treatment of 60 cases of gouty arthritis with modified Simiao Tang. J. Tradit. Chin. Med. = Chung I Tsa Chih Ying Wen Pan 2008, 28, 94–97. [Google Scholar]
- Han, J.; Shi, G.; Li, W.; Wang, S.; Bai, J.; Sun, X.; Xie, Y.; Sui, F.; Chen, F.; Jiang, D. Zisheng Shenqi Decoction Ameliorates Monosodium Urate-Mediated Gouty Arthritis in Rats via Promotion of Autophagy through the AMPK/mTOR Signaling Pathway. Evid.-Based Complementary Altern. Med. Ecam 2021, 2021, 6918026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Cheng, J.; Zhao, P.; Chen, K.L.; Li, J. Screening the Best Compatibility of Selaginella moellendorffii Prescription on Hyperuricemia and Gouty Arthritis and Its Mechanism. Evid.-Based Complementary Altern. Med. Ecam 2019, 2019, 7263034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, X.; Zhang, H.; Zhang, S.; Ma, K. Chinese herbal medicine for gout: A review of the clinical evidence and pharmacological mechanisms. Chin. Med. 2020, 15, 17. [Google Scholar] [CrossRef] [Green Version]
- Hong, Q.; Yu, S.; Mei, Y.; Lv, Y.; Chen, D.; Wang, Y.; Geng, W.; Wu, D.; Cai, G.; Chen, X. Smilacis Glabrae Rhizoma reduces oxidative stress caused by hyperuricemia via upregulation of catalase. Evid.-Based Complementary Altern. Med. Ecam 2014, 34, 1675–1685. [Google Scholar]
- Xu, W.A.; Yin, L.; Pan, H.Y.; Shi, L.; Xu, L.; Zhang, X.; Duan, J.A. Study on the correlation between constituents detected in serum from Rhizoma Smilacis Glabrae and the reduction of uric acid levels in hyperuricemia. J. Ethnopharmacol. 2013, 150, 747–754. [Google Scholar] [CrossRef]
- Guo, W.; Dong, H.; Wang, D.; Yang, B.; Wang, X. Separation of Seven Polyphenols from the Rhizome of Smilax glabra by Offline Two Dimension Recycling HSCCC with Extrusion Mode. Molecules 2018, 23, 505. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Li, J.; Cao, J.; Xu, Q.; Komatsu, K.; Namba, T. A new flavanone isolated from rhizoma smilacis glabrae and the structural requirements of its derivatives for preventing immunological hepatocyte damage. Planta Med. 1999, 65, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Ma, Y.; Peng, C.; Lu, J.; Huang, H.; Shu, J. Chemical Constituents from the Ethyl Acetate Effective Parts of Smilacis Glabrae Rhizoma. J. Chin. Med. Mater. 2021, 44, 79–83. [Google Scholar]
- Cao, H.; Pauff, J.M.; Hille, R. X-ray crystal structure of a xanthine oxidase complex with the flavonoid inhibitor quercetin. J. Nat. Prod. 2014, 77, 1693–1699. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, R.; Zhang, G.; Gong, D. Mechanistic insights into the inhibition of quercetin on xanthine oxidase. Int. J. Biol. Macromol. 2018, 112, 405–412. [Google Scholar] [CrossRef]
- Wang, Y.; Curtis-Long, M.J.; Lee, B.W.; Yuk, H.J.; Kim, D.W.; Tan, X.F.; Park, K.H. Inhibition of tyrosinase activity by polyphenol compounds from Flemingia philippinensis roots. Bioorg. Med. Chem. 2014, 22, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Joosten, L.A.B.; Crişan, T.O.; Bjornstad, P.; Johnson, R.J. Asymptomatic hyperuricaemia: A silent activator of the innate immune system. Nat. Rev. Rheumatol. 2020, 16, 75–86. [Google Scholar] [CrossRef]
- Zheng, Y.; Guan, H.; Zhou, X.; Xu, Y.; Fu, C.; Xiao, J.; Ye, Z. The association of renal tubular inflammatory and injury markers with uric acid excretion in chronic kidney disease patients. Int. Urol. Nephrol. 2020, 52, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, C.; Cherubini, A.; Ble, A.; Bos, A.J.; Maggio, M.; Dixit, V.D.; Lauretani, F.; Bandinelli, S.; Senin, U.; Ferrucci, L. Uric acid and inflammatory markers. Eur. Heart J. 2006, 27, 1174–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.Y.; Zhang, W.; Zhang, Y.; Du, X.Y. Effect of Smilax glabra Water-extracts on Serum Uric Acid, Triglyceride and Cholesterol in Hyperuricemia Rats. China Pharm. 2011, 22, 4439–4440. [Google Scholar]
- Farag, M.A.; Handoussa, H.; Fekry, M.I.; Wessjohann, L.A. Metabolite profiling in 18 Saudi date palm fruit cultivars and their antioxidant potential via UPLC-qTOF-MS and multivariate data analyses. Food Funct. 2016, 7, 1077–1086. [Google Scholar] [CrossRef]
- Braguine, C.G.; Costa, E.S.; Magalhães, L.G.; Rodrigues, V.; da Silva Filho, A.A.; Bastos, J.K.; Silva, M.L.; Cunha, W.R.; Januário, A.H.; Pauletti, P.M. Schistosomicidal evaluation of Zanthoxylum naranjillo and its isolated compounds against Schistosoma mansoni adult worms. Z. Fur Naturforschung. C J. Biosci. 2009, 64, 793–797. [Google Scholar] [CrossRef]
- Chideh, S.; Pilard, S.; Attoumbré, J.; Saguez, R.; Hassan-Abdallah, A.; Cailleu, D.; Wadouachi, A.; Baltora-Rosset, S. 5-O-caffeoylshikimic acid from Solanum somalense leaves: Advantage of centrifugal partition chromatography over conventional column chromatography. J. Sep. Sci. 2014, 37, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Andrade, C.; Gomes, N.G.M.; Andrade, P.B.; Gil-Izquierdo, A.; Pereira, D.M.; Suksungworn, R.; Duangsrisai, S.; Videira, R.A.; Valentão, P. Valorisation of kitul, an overlooked food plant: Phenolic profiling of fruits and inflorescences and assessment of their effects on diabetes-related targets. Food Chem. 2021, 342, 128323. [Google Scholar] [CrossRef]
- Sambanthamurthi, R.; Tan, Y.; Sundram, K.; Abeywardena, M.; Sambandan, T.G.; Rha, C.; Sinskey, A.J.; Subramaniam, K.; Leow, S.S.; Hayes, K.C.; et al. Oil palm vegetation liquor: A new source of phenolic bioactives. Br. J. Nutr. 2011, 106, 1655–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuoka, M. Chemical and toxicological studies on bracken fern, Pteridium aquilinum var. latiusculum. VI. Isolation of 5-O-caffeoylshikimic acid as an antithiamine factor. Chem. Pharm. Bull. 1982, 30, 3219–3224. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.L.; Zhu, W.; Wang, M.; Xu, X.J.; Lu, C.J. Antioxidant and Anti-Inflammatory Activities of Phenolic-Enriched Extracts of Smilax glabra. Evid.-Based Complementary Altern. Med. Ecam 2014, 2014, 910438. [Google Scholar] [CrossRef]
- Okamoto, K.; Kusano, T.; Nishino, T. Chemical nature and reaction mechanisms of the molybdenum cofactor of xanthine oxidoreductase. Curr. Pharm. Des. 2013, 19, 2606–2614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Zhang, G.; Liao, Y.; Pan, J.; Gong, D. Dietary Flavonoids as Xanthine Oxidase Inhibitors: Structure-Affinity and Structure-Activity Relationships. J. Agric. Food Chem. 2015, 63, 7784–7794. [Google Scholar] [CrossRef]
- Yang, T.H.; Yan, D.X.; Huang, X.Y.; Hou, B.; Ma, Y.B.; Peng, H.; Zhang, X.M.; Chen, J.J.; Geng, C.A. Termipaniculatones A-F, chalcone-flavonone heterodimers from Terminthia paniculata, and their protective effects on hyperuricemia and acute gouty arthritis. Phytochemistry 2019, 164, 228–235. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Zhao, M.; Li, Y.; Zhang, D.; Yang, Y.; Li, L. Natural Xanthine Oxidase Inhibitor 5-O-Caffeoylshikimic Acid Ameliorates Kidney Injury Caused by Hyperuricemia in Mice. Molecules 2021, 26, 7307. https://doi.org/10.3390/molecules26237307
Zhang D, Zhao M, Li Y, Zhang D, Yang Y, Li L. Natural Xanthine Oxidase Inhibitor 5-O-Caffeoylshikimic Acid Ameliorates Kidney Injury Caused by Hyperuricemia in Mice. Molecules. 2021; 26(23):7307. https://doi.org/10.3390/molecules26237307
Chicago/Turabian StyleZhang, Dong, Mojiao Zhao, Yumei Li, Dafang Zhang, Yong Yang, and Lijing Li. 2021. "Natural Xanthine Oxidase Inhibitor 5-O-Caffeoylshikimic Acid Ameliorates Kidney Injury Caused by Hyperuricemia in Mice" Molecules 26, no. 23: 7307. https://doi.org/10.3390/molecules26237307
APA StyleZhang, D., Zhao, M., Li, Y., Zhang, D., Yang, Y., & Li, L. (2021). Natural Xanthine Oxidase Inhibitor 5-O-Caffeoylshikimic Acid Ameliorates Kidney Injury Caused by Hyperuricemia in Mice. Molecules, 26(23), 7307. https://doi.org/10.3390/molecules26237307