1. Introduction
Trypanosoma and Leishmania parasites are the etiological agents for the Trypanosomiasis and leishmaniasis diseases that affect millions of people worldwide [
1]. The sand-fly is the vector for
Leishmania donovani, which is the etiological agent of visceral leishmaniasis in humans, while the tsetse fly transmits trypanosomes of
Trypanosoma brucei, which is the causative agent of human African trypanosomiasis (HAT), also known as sleeping sickness. According to the World Health Organization’s (WHO) statistics, there are 12 million people currently affected by leishmaniasis in 88 countries, and about 350 million people are at risk. Approximately 500,000 people in sub-Saharan Africa are infected annually with the African trypanosome (
T. brucei), leading to thousands of deaths [
1]. The socio-economic impact of the morbidity and mortality caused by these protozoan parasites is significant, particularly in tropical and subtropical countries [
2,
3].
In the last few decades, there has been an increased focus on developing treatments for African trypanosomiasis. Although several antitrypanosomal agents from plants have been characterized, great efforts are still needed to search for more antiparasitic compounds that have been evolutionarily derived from nature.
The bite of a tsetse fly is responsible for the transmission of T. brucei, which quickly adapts to the mammalian host and becomes the bloodstream form (BSF). The metabolism of host glucose through glycolysis is essential for the BSF parasite to successfully infect the host. In BSF parasites, the glycolysis of host glucose provides the sole source of ATP production. The transfer of a phosphoryl group from ATP to glucose in glycolysis is catalyzed by hexokinases (HK). T. brucei expresses two hexokinase genes encoding T. brucei hexokinase 1 (TbHK1) and 2 (TbHK2), enzymes which are essential for the BSF parasite.
The
Hypericum genus belonging to the Hypericaceae family has been reported to be a prolific source of various secondary metabolites, such as flavonoids, prenylated phloroglucinols, naphthodianthrones, and volatile oils, with a wide range of biological activities, such as antidepressant, antiseptic, wound-healing, antioxidant, antiviral, anti-inflammatory and antidiabetic. Recently, studies on several Hypericum species have reported their antimicrobial activity against a number of bacterial and fungal strains [
4,
5,
6].
The present study investigates the antileishmanial activity against L. donovani (promastigotes, axenic amastigotes and intracellular amastigotes in THP1 cells), and the antitrypanosomal activity against T. brucei, of the fractions and isolated compounds of Hypericum afrum Lam. Quercetin (1) and myricetin (4) were discovered to have higher activity against T. brucei. Further virtual studies were carried out on TbHK1 as a target enzyme for investigating the probable mechanism of quercetin’s and myricetin’s inhibition of parasitic growth. Quercetin and myricetin bind to TbHK1 proximal to the active site, and could be cytotoxic to the African Trypanosoma parasite as a result of TbHK1 inhibition.
2. Results
2.1. Chemistry
Compounds
1–
7 were identified as quercetin (
1), myricitrin (
2), biapigenin (
3), myricetin (
4), hyperoside (
5), myricetin-3-
O-β-
d-galactopyranoside (
6) and myricetin-3’-
O-β-
d-glucopyranoside (
7) [
7,
8,
9,
10,
11,
12,
13]. Compound
7 was isolated from the genus hypericum for the first time. All the compounds were tested in vitro for their antiprotozoal activity. Pentamidine was used as a positive control drug in the antileishmanial assays, while DMFO (α-difluoromethylornithine) was used as the positive control drug in the antitrypanosomal assay, which showed IC
50 and IC
90 values of 3.634 and 8.804 μM, respectively.
2.2. Antiprotozoal Activity
In this study, the in vitro antiprotozoal activities were evaluated for the
Hypericum afrum aerial parts’ fractions (CHCl
3, EtOAc and
n-butanol) and isolated pure compounds
1–
7 (
Figure 1; see also
Supplementary Materials) against
T. brucei. The fractions and pure compounds were also tested against
L. donovani promastigotes, axenic amastigotes and intracellular amastigotes in THP1 cells, for the determination of general toxicity using standard experimental procedures.
None of the fractions or pure compounds showed in vitro antileishmanial activities (
Table 1 and
Table 2). Regarding the antitrypanosomal activity, the chloroform, ethyl acetate and n-butanol fractions of
H. afrum showed significant inhibitory activity against
T. brucei culture, with IC
50 values of 12.35, 13.53 and 12.93 and with IC
90 values of 14.94, 19.31 and 18.67 µg/mL, respectively. Only compounds
1 and
4 from the ethyl acetate fraction were found to have antitrypanozomal activity. All the samples were simultaneously tested against THP1 cell for the determination of general cytotoxicity.
Compounds
1 and
4 showed potent activity against
T. brucei at IC
50 values of 7.52 and 5.71 and IC
90 values of 9.76 and 7.97 µM, respectively (
Table 1).
2.3. Molecular Modeling Study
It is worth noting that the bloodstream form of T. brucei is reliant on the glycolytic pathway to generate energy using host glucose as a sole source. Due to the limited homology between the host and T. brucei glycolytic enzymes, this makes Hexokinase (TbHK1) a good drug target. Inhibitors of TbHK1 are trypanocidal, with low expected side effects.
A BLAST homology search identified Hexokinase KlHxk1 of the
Kluyveromyces lactis (PDB Accession code: 3O08) as the most suitable template. KlHxk1 was crystalized with a high resolution of 2 Å. The target and template share 31% sequence identity and 51% similar amino acids, and only 3% gaps were detected. Using protein family information from PFAM, 14 amino acids were identified as having strictly conserved aligned positions of the Hexokinase family, with E-values of 1.7 × 10
−60, including Thr60, Gly84, Gly92, Arg97, Gly157, Phe158, Phe160, Ser161, Pro163, Leu174, Trp177, Lys179, Asp214 and Gly217 (
Figure 2). Three major pockets were detected by Fpocket, one of which overlaps with the possible sugar binding site (
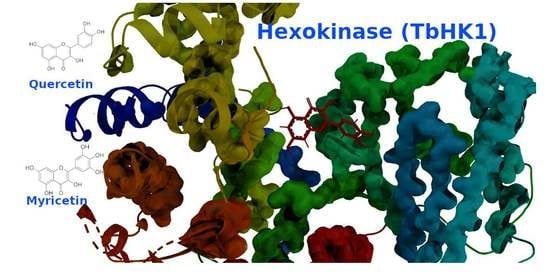
Figure 3). This site was selected for the docking of the compounds. Myricetin showed a docking score of −8.31 (and IFD score: −743.41), compared to −6.62 (IFD score: −735.48) for quercetin. The two compounds occupied the same pocket with very similar conformation (
Figure 4).
Quercetin exhibited hydrogen bonds with Ser161, Arg176, Thr178, Thr215 and Glu269, and π–π interaction with Phe162. The additional hydroxyl group in myricetin did not provide extra important interaction with the surrounding amino acids. On the other hand, some of the interactions were lost, which could be attributed to a slight shifting of the interacting functional groups of myricetin to allow for a proper placement of the additional hydroxyl group inside the binding site. Molecular dynamics (MD) simulations were conducted for 50 ns to study the stability and strength of ligand binding. The root means square deviation (RMSD) is used as an indicator for interaction stability.
N is the number of atoms in the system,
tref is the reference time,
r′i is the position of the selected atoms (i) in frame x,
tx is the record time of frame x.
Myricetin showed a stable pose in the binding pocket over the course of the MD time. It showed an RMSD value of ~3.0 Å, which is comparable to the 2.4 Å of the protein backbone, while quercetin showed a high RMSD value of ~15.0 Å compared to that of the protein backbone (2.9 Å). The binding mode of quercetin required the first 10 ns to adopt the most stable pose in the binding pocket (
Figure 5). The reference interaction site model (RISM) solvation approach was used to investigate the location and stability of water molecules (
Figure 6 and
Figure 7).
Possible water molecule locations were computed and thermodynamically analyzed. The absolute protein–ligand binding free energies were then calculated using the water swap method to rank quercetin and myricetin, by relying on the fact that protein–ligand binding is a competition between ligand and water for binding to the pocket (
Figure 8). Because this approach allows for the decomposition of ΔG on a per-residue basis, it permits the identification of which amino acid residues favor binding to ligand or water (
Figure 9). The free energy of binding is calculated in kcal/mol for each ligand with different methods, including Bennett, thermodynamic integration (TI), free energy perturbation (FEP) and TI Quadrature. The results in kcal/mol for myricetin are as follows: Bennett, −15.34; TI, −14.15; FEP, −14.14; and TI Quadrature, −14.23. Those of quercetin are Bennett, −9.92; TI, −9.28; FEP, –8.84; and TI Quadrature, −9.6. Then, a consensus-calculated free energy of binding was defined from a weighted arithmetic mean of the Bennett (50%), TI (30%), and FEP (20%) free energy estimators. The consensus is −14.74 for myricetin and −9.51 for quercetin.
3. Discussion
Flavonoids isolated from different natural resources were reported to exhibit moderate to high in vitro antitrypanosomal activity against T. brucei. Our results showed that the chloroform, ethyl acetate and n-butanol fractions of H. afrum exhibited potent antitrypanosomal activity against T. brucei, with IC50 values of 12.35, 13.53 and 12.93 and with IC90 values of 14.94, 19.31 and 18.67 µg/mL, respectively. Quercetin and myricetin were isolated from the EtOAc fraction, and showed good activity towards T. brucei, with IC50 values of 7.52 and 5.71, and IC90 values of 9.76 and 7.97 µM, respectively. A future examination of the chloroform and n-butanol fractions might yield bioactive molecules.
Quercetin and structurally related flavonoids possess several biological and pharmacological activities. Quercetin and myricetin are dietary flavonoids with promising activities, including antioxidant, anti-inflammatory, cardiovascular, and others [
14,
15]. The mechanism of action for quercetin and myricetin as antiparasitics has been postulated as follows: these flavonoids can disrupt the mitochondrial function on the parasites, and most likely inhibit different enzymes, including shock proteins, topoisomerases and kinases, among others. They could also show indirect activity through the induction of microbicidal responses, for example, the production of various cytokines and the production of nitric oxide [
16]. Hexokinase (TbHK1) enzymes have been shown to be promising targets on
T. brucei [
17,
18]. Quercetin and myricetin were identified as inhibitors of TbHK1, and showed IC
50 values of 4.1 and 48.9 µM, respectively [
16]. These results support the idea of considering TbHK1 as a target for antiparasitic activities. These results were further confirmed by our computational studies.
Quercetin has been reported as a potent antileishmanial agent; however, in our study, we did not find it [
19].
4. Material and Methods
4.1. General Experimental Procedures
Melting points were determined on an Opti-Melt automated melting point system (Stanford Research Systems), and were uncorrected. IR spectra were recorded using an Agilent model Cary 630 FT-IR. Optical rotations were recorded using a Rudolph Research Analytical Autopol V Polarimeter. UV was obtained using a Perkin–Elmer Lambda 3B UV/vis spectrophotometer.
1H- and
13C-NMR spectra were obtained on Bruker model AMX 500 and 400 NMR spectrometers with standard pulse sequences, operating at 500 and 400 MHz in
1H and 125 and 100 MHz in
13C. The coupling constants were recorded in Hertz (Hz). Standard pulse sequences were used for COSY, HMQC, HMBC and DEPT. All spectra were run at 25 °C. High-resolution mass spectra (HRMS) were measured on a Micromass Q-Tof Micro mass spectrometer with a lock spray source. Column chromatography was carried out on silica gel (70–230 mesh, Merck, Darmstadt, Germany), C18 SPE (500 mg Bed, Thermo scientific, Waltham, MA, USA), Diaion HP-20 (Sorbetch technologies, Norcross, GA, USA), MN-polyamide-SC-6 (Sigma Aldrich, St Louis, MO, USA) and Sephadex LH-20 (Sigma Aldrich, St Louis, MO, USA). TLC (silica gel 60 F254, EmD Millipore Sigma, Darmstadt, Germany) was used to monitor the fractions from column chromatography. Preparative TLC was carried out on silica gel 60 PF254 + 366 plates (20 cm × 20 cm, 1 mm thick). Visualization of the TLC plates was achieved with a UV lamp (λ = 254 and 365 nm) and an anisaldehyde/acid spray reagent (MeOH-acetic acid-anisaldehyde-sulfuric acid, 85:9:1:5). The absolute configurations of the sugar moieties of all tested flavonoid glycosides have been identified by the UPLC-UV/MS method [
20].
4.2. Plant Material
The aerial parts of Hypericum afrum (Lam.) were collected from the El Kala region, El Tarf, in northeastern Algeria, in July 2011. A voucher specimen (UM-10012014) was deposited in the culture collection of the Department of BioMolecular Sciences, University of Mississippi.
4.3. Extraction and Isolation
Extraction and isolation. Dried powdered aerial parts (1000 g) of H. afrum were macerated at room temperature with EtOH–H2O (80:20, v/v) over 24 h, three times. The filtered solvents were combined and evaporated under vacuum at a temperature of 40 °C to give a residue (30 g). The obtained extract was suspended in water (800 mL) and successively partitioned with CHCl3, EtOAc and n-butanol, yielding 500 mg (CHCl3), 7g (EtOAc) and 12g (n-butanol) fractions, respectively.
The EtOAc fraction (7 g) was chromatographed on a silica gel column and eluted with a CH2Cl2–MeOH solvent system of increasing polarity to yield 10 subfractions (E-1–10) according to their TLC behavior. The subfraction E-3 (115mg) was further chromatographed on the column of a Sephadex LH-20 with CH2Cl2–MeOH (1:1) as the eluent yielding compound 1 (16 mg) as a yellow precipitate. A yellow precipitate was obtained from the subfraction E-7 (40% MeOH in CH2Cl2). The solid was combined and subjected to a column of Sephadex LH-20 eluted with methanol to furnish compound 2 (7 mg). The subfraction E-4 (10% MeOH) (423 mg) was chromatographed on Sephadex LH-20 eluted with methanol to furnish compound 3 (10 mg). Subfraction E-5 (125 mg) was chromatographed on a Sephadex LH-20 column with Methanol and further purified by preparative TLC eluted with CHCl3–MeOH (10:1) to afford compound 4 (15 mg).
The n-butanol fraction (12 g) was subjected to column chromatography over Diaion HP-20 to afford three subfractions: A (H2O 100%), B (50% Me0H in water) and C (100% MeOH). Subfraction C (8 g) was subjected to polyamide column chromatography to give 13 subfractions (C-I to C-XIII). Then, 5 g of the subfraction C-X (100% methanol) was chromatographed on MN-polyamide-SC-6 CC (250 g), eluted with water to equilibrate, then with gradient–decreased polarities with a water–methanol system to yield 8 subfractions (C-X-1 to C-X-8). Subfraction C-X-4 (50 mg) was further subjected to a column of Sephadex LH-20 (20 g) eluted with MeOH-CH2Cl2 (1:1) to afford compound 5 (4.0 mg). C-X-5 (70 mg) was subjected to a column of Sephadex LH-20 (30 g) eluted with MeOH-CH2Cl2 (1:1) to afford compounds 6 (5.0 mg) and 7 (10.6 mg).
4.4. In Vitro Antileishmanial and Antitrypanosomal Assays
The in vitro antileishmanial and antitrypanosomal assays were performed on cell cultures of
L. donovani promastigotes, axenic amastigotes, THP1-amastigotes, and
Trypanosoma brucei trypomastigotes, by Alamar Blue assays [
21]. The conditions for seeding the THP1 cells, the exposure to the test samples and the evaluation of cytotoxicity were the same as described in the parasite rescue and transformation assay [
22]. IC
50 and IC
90 values were computed from the dose–response curves using XLfit software. DFMO (difluoromethylornithine) was used as the positive control. The antiprotozoal activities of the
H. afrum fractions and isolated compounds were evaluated in vitro against
L. donovani promastigotes, axenic amastigotes and intracellular amastigotes in THP1 cells. The fractions and some isolated compounds were also evaluated against
T. brucei trypomastigote forms. All the fractions and compounds were simultaneously tested against THP1 cell for the determination of general cytotoxicity [
22].
4.5. Methods: Molecular Modeling
The amino acid sequence of TbHK1 was downloaded from the Uniprot database (
www.uniprot.org)(accessed on 1 December 2019). Prime was used to build the 3D model of the target [
23,
24,
25]. To find the appropriate template, a BLAST homology search was initiated with default options. The globally conserved residues were also identified through the standard Prime search algorithm. The alignment between the target sequence and the template was calculated to define the complementary secondary structure information using the Prime STA option. An all-atom model was constructed using the knowledge-based method. Hydrogen bond assignment and restrained minimization options were used to fix and relax the generated model. Loop refinement was performed for the model to optimize the loops’ conformation. Fpocket [
26] was then used to detect the ligand binding sites in the model. We used Glide for the molecular docking step. The receptor grid was prepared by selecting the amino acids defining the pocket detected by Fpocket. To allow for more space for the ligands in the binding pocket, scaling of the van der Waals radii by a factor of 0.8 was employed to soften the potential of the nonpolar parts of the receptor. The prepared ligands were docked using Glide SP docking precision [
27,
28,
29,
30,
31]. The best-docked pose was saved for each ligand. Then, these poses were used for the molecular dynamics simulation [
32,
33], 3D-RISM [
34,
35,
36,
37] and WaterSwap [
38,
39] steps. The two complexes were solvated in an orthorhombic box using a TIP4P water solvation model in Desmond System Builder. Appropriate numbers of sodium ions were added to the system to neutralize the net charge on the protein. The OPLS3 force field was selected for the simulation run. After a series of energy minimizations and short simulations, the NPT ensemble was set for the MD production step. Intervals of 25 ps were used to save the coordinates and the MD simulations were set for 50 ns. The protein–ligand complex was prepared as recommended for the 3D-RISM calculation. The grid was defined for the whole protein and the calculation was performed using the XED force field. We used a grid spacing of 0.3 Å with a grid external width of 14.0 Å. The convergence tolerance was set to 10
−8 and the total formal charge was neutralized with counter ions. A WaterSwap calculation was conducted for the ligands. The normal calculation method was chosen. The charge method was set to Gasteiger, and the solvation box buffer dimension was defined as 10.0 Å. The system was equilibrated for 100 ps using OpenMM.
5. Conclusions
The in vitro antitrypanosomal evaluation of the Hypericum afrum Lam. species against T. brucei revealed that the CHCl3, EtOAc and BuOH fractions possess potent antitrypanosomal activity. Furthermore, the fractionation of these fractions led to the isolation and characterization of quercetin and myricetin as the potent components. Quercetin (1) and myricetin (4) showed good antitrypanosomal activity towards T. brucei, with IC50 and IC90 values of 7.52 and 5.71, and 9.76 and 7.97 µM, respectively.
The present study reports for the first time the antiprotozoal properties of the Algerian species Hypericum afrum Lam., contributing to the phytochemical and pharmacological knowledge of this species. Moreover, this species could be a source of new antitrypanosomal agents.
The mechanism of antitrypanosomal activity was investigated using molecular docking studies on the potential target enzyme T. brucei Hexokinase (TbHK1). Docking studies of molecules (1) and (4) revealed their strong affinity towards Hexokinase (TbHK1) as a target of T. brucei.
Supplementary Materials
The following are available online, Figure S1: 1H-NMR spectrum of compound 1, Figure S2: 13C-NMR spectrum of compound 1, Figure S3: Positive HRESIMS of compound 1, Figure S4: 1H-NMR spectrum of compound 2, Figure S5: 13C-NMR spectrum of compound 2, Figure S6: Negative HRESIMS of compound 2, Figure S7: 1H-NMR spectrum of compound 3, Figure S8: 13C-NMR spectrum of compound 3, Figure S9: Positive HRESIMS of compound 3, Figure S10: 1H-NMR spectrum of compound 4, Figure S11. 13C-NMR spectrum of compound 4, Figure S12: Negative HRESIMS of compound 4. Figure S13: 1H-NMR spectrum of compound 5, Figure S14: 13C-NMR spectrum of compound 5, Figure S15: Negative HRESIMS of compound 5, Figure S16: 1H-NMR spectrum of compound 6, Figure S17: 13C-NMR spectrum of compound 6, Figure S18: Negative HRESIMS of compound 6, Figure S19: 1H-NMR spectrum of compound 7, Figure S20: 13C-NMR spectrum of compound 7, Figure S21: Negative HRESIMS of compound 7.
Author Contributions
Conceptualization, F.L. (Farida Larit), K.M.E., M.A.N. and F.L. (Francisco León); methodology, F.L. (Farida Larit), K.M.E., M.A.N. and F.L. (Francisco León); software, F.L. (Farida Larit), K.M.E., M.A.N. and F.L. (Francisco León); validation, F.L. (Farida Larit), K.M.E., M.A.N., M.M.G. and F.L. (Francisco León); formal analysis, F.L. (Farida Larit), K.M.E., M.A.N. and F.L. (Francisco León); investigation, F.L. (Farida Larit), K.M.E., M.A.N. and F.L.(Francisco León); resources, F.L. (Farida Larit), K.M.E., M.A.N., F.L. (Francisco León), M.M.G., S.J.C.; data curation, F.L. (Farida Larit), K.M.E., M.A.N.; writing—original draft preparation, F.L. (Farida Larit), K.M.E., M.A.N.; writing—review and editing, F.L. (Farida Larit), K.M.E., M.A.N., M.M.G. and F.L. (Francisco León); visualization, F.L. (Farida Larit), K.M.E., M.A.N. and F.L. (Francisco León); supervision, F.L. (Farida Larit), K.M.E., M.A.N., F.L. (Francisco León) and S.J.C.; project administration, F.L. (Farida Larit), S.B., S.J.C. and F.L. (Francisco León); funding acquisition, M.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by grant numbers P20GM104932 and P30GM122733 from the National Institute of General Medical Sciences (NIGMS), a component of the National Institutes of Health (NIH), and was conducted in part in a facility constructed with support from the Research Facilities Improvements Program (C06RR14503) from the NIH National Center for Research Resources; its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIGMS or NIH.
Data Availability Statement
Data is available from the authors.
Acknowledgments
We are grateful to the Algerian Ministry of Higher Education and Scientific Research and the University of Mississippi, School of Pharmacy, Mississippi, USA and AlMaarefa University for their financial support. We are especially thankful to Babu Tekwani and Melissa Jacob for providing the antiprotozoal assays.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 1–7 are available from the authors.
References
- WHO. Available online: https://www.who.int/leishmaniasis/en/ (accessed on 23 December 2020).
- Okwor, I.; Uzonna, J. Social and economic burden of human leishmaniasis. Am. J. Trop. Med. Hyg. 2016, 94, 489–493. [Google Scholar] [CrossRef] [Green Version]
- Boelaert, M.; Meheus, F.; Robays, J.; Lutumba, P. Socio–economic aspects of neglected diseases: Sleeping sickness and visceral leishmaniasis. Ann. Trop. Med. Parasitol. 2010, 104, 535–542. [Google Scholar] [CrossRef]
- Larit, F.; León, F.; Jasika-Misiak, I.; Wieczorek, P.; Cutler, S. Phenolic Content and Antioxidant Activity of Cytisus Villosus and Hypericum Afrum Extracts. Planta Med. 2016, 82, PB21. [Google Scholar] [CrossRef]
- Larit, F.; León, F.; Chaurasiya, N.D.; Tekwani, B.L.; Benyahia, S.; Benayache, S.; Cutler, S.J. A new phloroglucinol derivative isolated from Hypericum afrum, a plant endemic to Algeria. Rec. Nat. Prod. 2017, 11, 77–81. [Google Scholar]
- Larit, F.; Elokely, K.M.; Chaurasiya, N.D.; Benyahia, S.; Nael, M.A.; León, F.; Abu-Darwish, M.S.; Efferth, T.; Wang, Y.-H.; Belouahem-Abed, D. Inhibition of human monoamine oxidase A and B by flavonoids isolated from two Algerian medicinal plants. Phytomedicine 2018, 40, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, F.; Cottiglia, F.; Bonsignore, L.; Casu, L.; Casu, M.; Floris, C.; Secci, D.; Bolasco, A.; Chimenti, P.; Granese, A. Quercetin as the active principle of Hypericum hircinum exerts a selective inhibitory activity against MAO-A: Extraction, Biological analysis, and computational study. J. Nat. Prod. 2006, 69, 945–949. [Google Scholar] [CrossRef]
- Eldahshan, O.A. Isolation and structure elucidation of phenolic compounds of carob leaves grown in Egypt. Curr. Res. J. Biol. Sci. 2011, 3, 52–55. [Google Scholar]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- David, J.M.; Cruz, F.G.; Guedes, M.L.S.; Chávez, J.P. Flavonol glycosides from Davilla flexuosa. J. Braz. Chem. Soc. 1996, 7, 115–118. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, S.D.; Li, P. Determination of flavonoids in Hypericum perforatum by HPLC analysis. Yao Xue Xue Bao Acta Pharm. Sin. 2002, 37, 280–282. [Google Scholar]
- Paul, B.; Rao, G.S.; Kapadia, G.J. Isolation of myricadiol, myricitrin, taraxerol, and taraxerone from Myrica cerifera L. root bark. J. Pharm. Sci. 1974, 63, 958–959. [Google Scholar] [CrossRef]
- Baoliang, C.; Motoyuki, N.; Junei, K.; Toshihiro, N. Chemical constituents of Astragali semen. Chem. Pharm. Bull. 1993, 41, 178–182. [Google Scholar]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A dietary molecule with diverse biological activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 20, 11849–11872. [Google Scholar] [CrossRef] [PubMed]
- Dodson, H.C.; Lyda, T.A.; Chambers, J.W.; Morris, M.T.; Christensen, K.A.; Morris, J.C. Quercetin, a fluorescent bioflavanoid, inhibits Trypanosoma brucei hexokinase 1. Exp. Parasitol. 2011, 127, 423–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coley, A.F.; Dodson, H.C.; Morris, M.T.; Morris, J.C. Glycolysis in the african trypanosome: Targeting enzymes and their subcellular compartments for therapeutic development. Mol. Biol. Int. 2011, 2011, 123702. [Google Scholar] [CrossRef] [Green Version]
- Sharlow, E.R.; Lyda, T.A.; Dodson, H.C.; Mustata, G.; Morris, M.T.; Leimgruber, S.S.; Lee, K.-H.; Kashiwada, Y.; Close, D.; Lazo, J.S. A target-based high throughput screen yields Trypanosoma brucei hexokinase small molecule inhibitors with antiparasitic activity. PLoS Negl. Trop. Dis. 2010, 4, e659. [Google Scholar] [CrossRef] [Green Version]
- Mittra, B.; Saha, A.; Chowdhury, A.R.; Pal, C.; Mandal, S.; Mukhopadhyay, S.; Bandyopadhyay, S.; Majumder, H.K. Luteolin, an abundant dietary component is a potent anti-leishmanial agent that acts by inducing topoisomerase II-mediated kinetoplast DNA cleavage leading to apoptosis. Mol. Med. 2000, 6, 527–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-H.; Avula, B.; Fu, X.; Wang, M.; Khan, I.A. Simultaneous Determination of the Absolute Configuration of Twelve Monosaccharide Enantiomers from Natural Products in a Single Injection by a UPLC-UV/MS Method. Planta Med 2012, 78, 834–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manda, S.; Khan, S.I.; Jain, S.K.; Mohammed, S.; Tekwani, B.L.; Khan, I.A.; Vishwakarma, R.A.; Bharate, S.B. Synthesis, antileishmanial and antitrypanosomal activities of N-substituted tetrahydro-β-carbolines. Bioorganic Med. Chem. Lett. 2014, 24, 3247–3250. [Google Scholar] [CrossRef]
- Jain, S.K.; Sahu, R.; Walker, L.A.; Tekwani, B.L. A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line. JoVE (J. Vis. Exp.) 2012, 70, e4054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobson, M.P.; Pincus, D.L.; Rapp, C.S.; Day, T.J.; Honig, B.; Shaw, D.E.; Friesner, R.A. A hierarchical approach to all-atom protein loop prediction. Proteins Struct. Funct. Bioinform. 2004, 55, 351–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobson, M.P.; Friesner, R.A.; Xiang, Z.; Honig, B. On the role of the crystal environment in determining protein side-chain conformations. J. Mol. Biol. 2002, 320, 597–608. [Google Scholar] [CrossRef]
- Release, S. 2: Maestro; Version 11.8; Schrödinger, LLC.: New York, NY, USA, 2018. [Google Scholar]
- Le Guilloux, V.; Schmidtke, P.; Tuffery, P. Fpocket: An open source platform for ligand pocket detection. BMC Bioinform. 2009, 10, 168. [Google Scholar] [CrossRef] [Green Version]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [Green Version]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Release, S. 2: Glide; Schrödinger, LLC.: New York, NY, USA, 2018. [Google Scholar]
- Bowers, K.J.; Chow, D.E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D. Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proceedings of the SC’06: 2006 ACM/IEEE Conference on Supercomputing, New York, NY, USA, 11–17 November 2006; p. 43. [Google Scholar]
- Shaw Research. Schrödinger Release 2019-2: Desmond Molecular Dynamics System; Shaw Research: New York, NY, USA, 2019. [Google Scholar]
- Schrödinger. Desmond Interoperability Tools; Schrödinger: New York, NY, USA, 2019. [Google Scholar]
- Skyner, R.; McDonagh, J.; Groom, C.; Van Mourik, T.; Mitchell, J. A review of methods for the calculation of solution free energies and the modelling of systems in solution. Phys. Chem. Chem. Phys. 2015, 17, 6174–6191. [Google Scholar] [CrossRef] [Green Version]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinter, J.G. Extended electron distributions applied to the molecular mechanics of some intermolecular interactions. J. Comput. Aided Mol. Des. 1994, 8, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Skylaris, C.-K.; Haynes, P.D.; Mostofi, A.A.; Payne, M.C. Introducing ONETEP: Linear-scaling density functional simulations on parallel computers. J. Chem. Phys. 2005, 122, 084119. [Google Scholar] [CrossRef] [PubMed]
- Woods, C.J.; Malaisree, M.; Hannongbua, S.; Mulholland, A.J. A water-swap reaction coordinate for the calculation of absolute protein–ligand binding free energies. J. Chem. Phys. 2011, 134, 02B611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, C.J.; Malaisree, M.; Michel, J.; Long, B.; McIntosh-Smith, S.; Mulholland, A.J. Rapid decomposition and visualisation of protein–ligand binding free energies by residue and by water. Faraday Discuss. 2014, 169, 477–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).