Improved Dermal and Transdermal Delivery of Curcumin with SmartFilms and Nanocrystals
Abstract
:1. Introduction
2. Results and Discussion
2.1. Production and Characterization of Curcumin-Loaded SmartFilms and Benchmark Controls
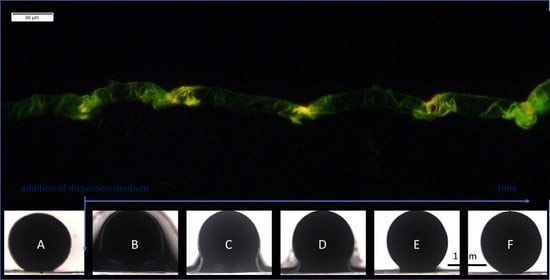
2.1.1. Curcumin-Loaded SmartFilms
2.1.2. Curcumin Bulk- and Nanosuspensions
2.1.3. Dissolution Behavior
2.2. Determination of Dermal Penetration Efficacy—Ex Vivo
2.3. Determination of Dermal Penetration Efficacy—Digital Image Analysis
2.4. Determination of Dermal Penetration Efficacy—Franz Diffusion Cells
2.5. A Step Forward to Understanding Passive Dermal Penetration of AIs from Particle Containing Topical Formulations
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Production of SmartFilms
3.2.2. Production of Curcumin Bulk- and Nanosuspensions
3.2.3. Characterization
Determination of Crystalline State
Determination of Curcumin Content
Determination of Dissolution Profile
Size Characterization of Curcumin Suspensions
3.2.4. Determination of Dermal Penetration Efficacy of Curcumin
In Vitro Testing with Franz Diffusion Cells
Ex Vivo Skin Penetration Studies
Epifluorescence Microscopy
Digital Image Analysis
3.2.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [Green Version]
- Cornier, J.; Keck, C.M.; van der Voorde, M. (Eds.) Nanocosmetics—From Ideas to Products; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharma. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef] [Green Version]
- Junyaprasert, V.B.; Morakul, B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015, 10, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Stegemann, S.; Leveiller, F.; Franchi, D.; de Jong, H.; Linden, H. When poor solubility becomes an issue: From early stage to proof of concept. Eur. J. Pharm. Sci. 2007, 31, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Fahr, A.; Liu, X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin. Drug Deliv. 2007, 4, 403–416. [Google Scholar] [CrossRef]
- Heimbach, T.; Fleisher, D.; Kaddoumi, A. Overcoming Poor Aqueous Solubility of Drugs for Oral Delivery. In Prodrugs; Stella, V.J., Borchardt, R.T., Hageman, M.J., Oliyai, R., Maag, H., Tilley, J.W., Eds.; Springer: New York, NY, USA, 2007; pp. 157–215. ISBN 978-0-387-49782-2. [Google Scholar]
- Lemke, S. Cellulosebasierte Filme (smartFilms®) als Alternative Orale Oder Perorale Applikationsform; Herstellung und Prüfung. Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany, 2017. [Google Scholar]
- Lemke, S.; Strätling, E.-J.; Welzel, H.-P.; Keck, C.M. Cellulose Fibre Based Support Matrices for Layered Products for Oral and Peroral Application and Their Preparation. EP Patent 3,192,499,A1, 14 January 2016. [Google Scholar]
- Stumpf, F.; Keck, C.M. Tablets made from paper. Int. J. Pharm. 2018, 548, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, F.; Keck, C.M. Tabletten aus Papier. Pharm. Ind. 2019, 2019, 392. [Google Scholar]
- Stumpf, F. Tabletten aus Papier-Tablets Made from Paper-Zur Oralen Applikation Schwerlöslicher Wirkstoffe. Ph.D. Thesis, Philipps-Universität Marburg, Marburg, Germany, 2019. [Google Scholar]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Jantarat, C.; Sirathanarun, P.; Boonmee, S.; Meechoosin, W.; Wangpittaya, H. Effect of Piperine on Skin Permeation of Curcumin from a Bacterially Derived Cellulose-Composite Double-Layer Membrane for Transdermal Curcumin Delivery. Sci. Pharm. 2018, 86, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paolino, D.; Vero, A.; Cosco, D.; Pecora, T.M.G.; Cianciolo, S.; Fresta, M.; Pignatello, R. Improvement of Oral Bioavailability of Curcumin upon Microencapsulation with Methacrylic Copolymers. Front. Pharmacol. 2016, 7, 485. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Ji, H.-F. The pharmacology of curcumin: Is it the degradation products? Trends Mol. Med. 2012, 18, 138–144. [Google Scholar] [CrossRef]
- Tejada, S.; Manayi, A.; Daglia, M.; Nabavi, S.F.; Sureda, A.; Hajheydari, Z.; Gortzi, O.; Pazoki-Toroudi, H.; Nabavi, S.M. Wound Healing Effects of Curcumin: A Short Review. Curr. Pharm. Biotechnol. 2016, 17, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Santezi, C.; Reina, B.D.; Dovigo, L.N. Curcumin-mediated Photodynamic Therapy for the treatment of oral infections—A review. Photodiagn. Photodyn. Ther. 2018, 21, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Donsì, F.; Wang, Y.; Li, J.; Huang, Q. Preparation of curcumin sub-micrometer dispersions by high-pressure homogenization. J. Agric. Food Chem. 2010, 58, 2848–2853. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S. Design of Cilostazol Nanocrystals for Improved Solubility. J. Pharm. Innov. 2020, 15, 416–423. [Google Scholar] [CrossRef]
- Gigliobianco, M.R.; Casadidio, C.; Censi, R.; Di Martino, P. Nanocrystals of Poorly Soluble Drugs: Drug Bioavailability and Physicochemical Stability. Pharmaceutics 2018, 10, 134. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Siril, P.F. Enhancing the Solubility of Fenofibrate by Nanocrystal Formation and Encapsulation. AAPS PharmSciTech 2018, 19, 284–292. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Wang, L.; Yan, B.; Gu, Y.; Chang, P.; Wang, Y. Nanocrystals Technology for Transdermal Delivery of Water-Insoluble Drugs. Curr. Drug Deliv. 2018, 15, 1221–1229. [Google Scholar] [CrossRef]
- Pawar, V.K.; Singh, Y.; Meher, J.G.; Gupta, S.; Chourasia, M.K. Engineered nanocrystal technology: In-vivo fate, targeting and applications in drug delivery. J. Control. Release 2014, 183, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Allen, T. Particle Size Measurement, 4th ed.; Springer: Dordrecht, The Netherlands, 1990; ISBN 9789401066730. [Google Scholar]
- Pelikh, O.; Pinnapireddy, S.R.; Keck, C.M. Dermal penetration analysis of curcumin in an ex-vivo porcine ear model using epifluorescence microscopy and digital image processing. Skin Pharmacol. Physiol. 2021. [Google Scholar] [CrossRef]
- Lademann, J.; Richter, H.; Meinke, M.; Sterry, W.; Patzelt, A. Which skin model is the most appropriate for the investigation of topically applied substances into the hair follicles? Skin Pharmacol. Physiol. 2010, 23, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Richter, H.; Knorr, F.; Patzelt, A.; Darvin, M.E.; Rühl, E.; Cheung, K.Y.; Lai, K.K.; Renneberg, R.; Mak, W.C. Triggered release of model drug from AuNP-doped BSA nanocarriers in hair follicles using IRA radiation. Acta Biomater. 2016, 30, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.B.; Benfeldt, E.; Holmgaard, R. Penetration through the Skin Barrier. Curr. Probl. Dermatol. 2016, 49, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Jamison, J.A.; Krueger, K.M.; Yavuz, C.T.; Mayo, J.T.; LeCrone, D.; Redden, J.J.; Colvin, V.L. Size-dependent sedimentation properties of nanocrystals. ACS Nano 2008, 2, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Franz, T.J. Percutaneous absorption on the relevance of in vitro data. J. Investig. Dermatol. 1975, 64, 190–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra-Ponce, W.L.; Gracia-Vásquez, S.L.; González-Barranco, P.; Camacho-Mora, I.A.; Gracia-Vásquez, Y.A.; Orozco-Beltrán, E.; Felton, L.A. In vitro evaluation of sustained released matrix tablets containing ibuprofen: A model poorly water-soluble drug. Braz. J. Pharm. Sci. 2016, 52, 751–759. [Google Scholar] [CrossRef]
- Salamanca, C.H.; Barrera-Ocampo, A.; Lasso, J.C.; Camacho, N.; Yarce, C.J. Franz Diffusion Cell Approach for Pre-Formulation Characterisation of Ketoprofen Semi-Solid Dosage Forms. Pharmaceutics 2018, 10, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakarinen, O.H.; Foster, A.S.; Paajanen, M.; Kalinainen, T.; Katainen, J.; Makkonen, I.; Lahtinen, J.; Nieminen, R.M. Towards an accurate description of the capillary force in nanoparticle-surface interactions. Model. Simul. Mater. Sci. Eng. 2005, 13, 1175–1186. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Elsevier: Amsterdam, The Netherlands; Elsevier: Boston, MA, USA; Elsevier: Paris, France, 2011; ISBN 9780123751829. [Google Scholar]
- Pelikh, O.; Keck, C.M. Hair Follicle Targeting and Dermal Drug Delivery with Curcumin Drug Nanocrystals-Essential Influence of Excipients. Nanomaterials 2020, 10, 2323. [Google Scholar] [CrossRef]
- Ali, Z.; Saleem, M.; Atta, B.M.; Khan, S.S.; Hammad, G. Determination of curcuminoid content in turmeric using fluorescence spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 213, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Moustapha, A.; Pérétout, P.A.; Rainey, N.E.; Sureau, F.; Geze, M.; Petit, J.-M.; Dewailly, E.; Slomianny, C.; Petit, P.X. Curcumin induces crosstalk between autophagy and apoptosis mediated by calcium release from the endoplasmic reticulum, lysosomal destabilization and mitochondrial events. Cell Death Discov. 2015, 1, 15017. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Ha, P.T.; Nguyen, A.S.; Nguyen, D.T.; Do, H.D.; Thi, Q.N.; Thi, M.N.H. Curcumin as fluorescent probe for directly monitoring in vitro uptake of curcumin combined paclitaxel loaded PLA-TPGS nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 25001. [Google Scholar] [CrossRef]
- Romero, G.B.; Keck, C.M.; Müller, R.H. Simple low-cost miniaturization approach for pharmaceutical nanocrystals production. Int. J. Pharm. 2016, 501, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Keck, C.M. Cyclosporine Nanosuspensions: Optimised Size Characterisation & Oral Formulations. Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany, 2006. [Google Scholar]
- Radtke, M. Grundlegende Untersuchung zur Arzneistoffinkorporation, Freisetzung und Struktur von SLN und NLC. Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany, 2003. [Google Scholar]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of Curcumin: From Mechanism to Biological Implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, W.M.; Ng, K.W. Finite and Infinite Dosing. In Percutaneous Penetration Enhancers Drug Penetration Into/Through the Skin; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 35–44. ISBN 978-3-662-53268-3. [Google Scholar]
- Binder, L.; Mazál, J.; Petz, R.; Klang, V.; Valenta, C. The role of viscosity on skin penetration from cellulose ether-based hydrogels. Skin Res. Technol. 2019, 25, 725–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasband, W.S. ImageJ: Image Processing and Analysis in Java; Astrophysics Source Code Library: Houghton, MI, USA, 2012. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Pelikh, O.; Eckert, R.W.; Pinnapireddy, S.R.; Keck, C.M. Hair follicle targeting with curcumin nanocrystals: Influence of the formulation properties on the penetration efficacy. J. Control. Release 2020, 329, 598–613. [Google Scholar] [CrossRef]
- JASP Team. JASP-Software (Version 0.13.1). 2020. Available online: https://jasp-stats.org/2020/07/02/introducing-jasp-0-13/ (accessed on 15 March 2021).
- Dinno, A. Nonparametric pairwise multiple comparisons in independent groups using Dunn’s test. Stata J. 2015, 15, 292–300. [Google Scholar] [CrossRef] [Green Version]









| SmartFilms | Bulk Suspension Non-Diluted | Nanosuspension Non-Diluted | Bulk Suspension Diluted | Nanosuspension Diluted | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| curcumin content [µg] in formulation | 684 | ± | 16 | 2098 * | ± | 41 | 1946 * | ± | 48 | 574 * | ± | 99 | 614 * | ± | 11 |
| z-average [nm] | n.a. | n.a. | 217 | ± | 0.1 | n.a. | 205 | ± | 0.1 | ||||||
| PDI | 0.31 | ± | 0.02 | 0.35 | ± | 0.02 | |||||||||
| d(v)0.1 [µm] | 11 | ± | 0.5 | 0.02 | ± | 0.0 | 8 | ± | 0.3 | 0.02 | ± | 0.0 | |||
| d(v)0.5 [µm] | 45 | ± | 7 | 0.1 | ± | 0.0 | 24 | ± | 2 | 0.1 | ± | 0.0 | |||
| d(v)0.9 [µm] | 153 | ± | 58 | 0.2 | ± | 0.0 | 65 | ± | 9 | 0.2 | ± | 0.0 | |||
| d(v)0.95 [µm] | 188 | ± | 77 | 0.5 | ± | 0.0 | 84 | ± | 15 | 0.5 | ± | 0.0 | |||
| d(v)0.99 [µm] | 251 | ± | 113 | 2.5 | ± | 0.2 | 130 | ± | 32 | 2.2 | ± | 0.1 | |||
| amount of curcumin [µg/cm2] applied on skin | 152 | 466 | 432 | 128 | 136 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckert, R.W.; Wiemann, S.; Keck, C.M. Improved Dermal and Transdermal Delivery of Curcumin with SmartFilms and Nanocrystals. Molecules 2021, 26, 1633. https://doi.org/10.3390/molecules26061633
Eckert RW, Wiemann S, Keck CM. Improved Dermal and Transdermal Delivery of Curcumin with SmartFilms and Nanocrystals. Molecules. 2021; 26(6):1633. https://doi.org/10.3390/molecules26061633
Chicago/Turabian StyleEckert, Ralph W., Sabrina Wiemann, and Cornelia M. Keck. 2021. "Improved Dermal and Transdermal Delivery of Curcumin with SmartFilms and Nanocrystals" Molecules 26, no. 6: 1633. https://doi.org/10.3390/molecules26061633
APA StyleEckert, R. W., Wiemann, S., & Keck, C. M. (2021). Improved Dermal and Transdermal Delivery of Curcumin with SmartFilms and Nanocrystals. Molecules, 26(6), 1633. https://doi.org/10.3390/molecules26061633