Umbu Fruit Peel as Source of Antioxidant, Antimicrobial and α-Amylase Inhibitor Compounds
Abstract
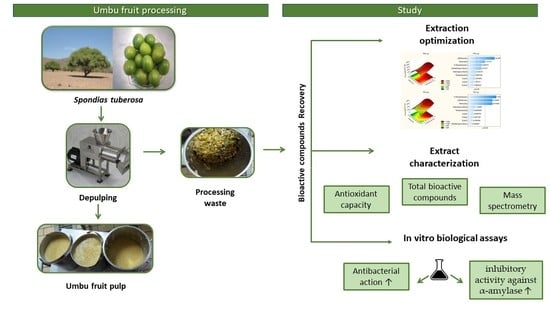
:1. Introduction
2. Results and Discussion
2.1. Effect of Independent Variables
2.2. Selection of the Optimal Operational Condition
2.3. Bioactive Profile by LC-HRMS
2.4. Antimicrobial Assays
2.5. α-Amylase Inhibition
3. Materials and Methods
3.1. Umbu Fruit Peel
3.2. Thermal-Assisted Solid–Liquid Extraction
3.3. Experimental Design
3.4. Chemical Analysis
3.4.1. Total Phenolic Compounds (TPC)
3.4.2. Total Flavonoid Compounds (TFC)
3.4.3. ABTS•+ Assay
3.4.4. DPPH• Assay
3.4.5. FRAP Assay
3.4.6. UPLC-qTOF/MS Analysis
3.5. In Vitro Biological Studies
3.5.1. Antimicrobial Assays
3.5.2. Assay for α-Amylase Inhibition
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Genovese, M.I.; da Silva Pinto, M.; de Souza Schmidt Goncalves, A.E.; Lajolo, F.M. Bioactive Compounds and Antioxidant Capacity of Exotic Fruits and Commercial Frozen Pulps from Brazil. Food Sci. Technol. Int. 2008, 14, 207–214. [Google Scholar] [CrossRef]
- Santamarina, A.B.; Jamar, G.; Mennitti, L.V.; Ribeiro, D.A.; Cardoso, C.M.; de Rosso, V.V.; Oyama, L.M.; Pisani, L.P. Polyphenols-Rich Fruit (Euterpe edulis Mart.) Prevents Peripheral Inflammatory Pathway Activation by the Short-Term High-Fat Diet. Molecules 2019, 24, 1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, L.O.; Viana, E.S.; Godoy, R.L.O.; de Freitas, S.C.; Freitas, S.P.; da Matta, V.M. Nutrients and bioactive compounds of pulp, peel and seed from umbu fruit. Cienc. Rural 2019, 49, e20180806. [Google Scholar] [CrossRef] [Green Version]
- IBGE. Produção da Extração Vegetal e da Silvicultura—PEVS 2018: Quantidade Produzida na Extração Vegetal; IBGE: Rio de Janeiro, Brazil, 2018. Available online: https://sidra.ibge.gov.br/pesquisa/pevs/quadros/brasil/2018 (accessed on 10 February 2020).
- Ribeiro, L.O.; Pontes, S.M.; Ribeiro, A.P.O.; Pacheco, S.; Freitas, S.P.; da Matta, V.M. Avaliação do armazenamento a frio sobre os compostos bioativos e as características físico-químicas e microbiológicas do suco de umbu pasteurizado. Braz. J. Food Technol. 2017, 20, e201509. [Google Scholar] [CrossRef]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.L.; Mazzutti, S.; de Souza, J.A.L.; Ferreira, S.R.S.; Soares, L.A.L.; Stragevitch, L.; Danielski, L. Extraction of umbu (Spondias tuberosa) seed oil using CO2, ultrasound and conventional methods: Evaluations of composition profiles and antioxidant activities. J. Supercrit. Fluids 2019, 145, 10–18. [Google Scholar] [CrossRef]
- Omena, C.M.B.; Valentim, I.B.; Guedes, G.d.S.; Rabelo, L.A.; Mano, C.M.; Bechara, E.J.H.; Sawaya, A.C.H.F.; Trevisan, M.T.S.; da Costa, J.G.; Ferreira, R.C.S.; et al. Antioxidant, anti-acetylcholinesterase and cytotoxic activities of ethanol extracts of peel, pulp and seeds of exotic Brazilian fruits. Antioxidant, anti-acetylcholinesterase and cytotoxic activities in fruits. Food Res. Int. 2012, 49, 334–344. [Google Scholar] [CrossRef] [Green Version]
- Cangussu, L.B.; Fronza, P.; Franca, A.S.; Oliveira, L.S. Chemical Characterization and Bioaccessibility Assessment of Bioactive Compounds from Umbu (Spondias tuberosa A.) Fruit Peel and Pulp Flours. Foods 2021, 10, 2597. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.V.; Costa, S.C.C.; Branco, C.R.C.; Branco, A. In vitro photoprotective activity of the Spondias purpurea L. peel crude extract and its incorporation in a pharmaceutical formulation. Ind. Crops Prod. 2016, 83, 509–514. [Google Scholar] [CrossRef]
- Gull, A.; Bhat, N.; Wani, S.M.; Masoodi, F.A.; Amin, T.; Ganai, S.A. Shelf life extension of apricot fruit by application of nanochitosan emulsion coatings containing pomegranate peel extract. Food Chem. 2021, 349, 129149. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Ribeiro, L.O.; Conrado Thomaz, G.F.; de Brito, M.; de Figueiredo, N.; Przytyk Jung, E.; Norie Kunigami, C. Siriguela peels provide antioxidant compounds-rich extract by solid–liquid extraction. J. Food Process. Preserv. 2020, 44, e14719. [Google Scholar] [CrossRef]
- Jesus, M.S.; Genisheva, Z.; Romaní, A.; Pereira, R.N.; Teixeira, J.A.; Domingues, L. Bioactive compounds recovery optimization from vine pruning residues using conventional heating and microwave-assisted extraction methods. Ind. Crops Prod. 2019, 132, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Chapter 15—Extraction of Polyphenols from Aromatic and Medicinal Plants: An Overview of the Methods and the Effect of Extraction Parameters. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 243–259. ISBN 978-0-12-813768-0. [Google Scholar]
- Ruíz-García, Y.; Beres, C.; Chávez, D.W.H.; Souza, E.F.; Tonon, R.V.; Cabral, L.M.C. Influence of processing conditions on bioactive compound extraction from Vitis vinifera L. var. Alicante Bouschet grape skin. J. Food Sci. Technol. 2019, 56, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Markom, M.; Hasan, M.; Daud, W.R.W.; Singh, H.; Jahim, J.M. Extraction of hydrolysable tannins from Phyllanthus niruri Linn.: Effects of solvents and extraction methods. Sep. Purif. Technol. 2007, 52, 487–496. [Google Scholar] [CrossRef]
- Engels, C.; Gräter, D.; Esquivel, P.; Jiménez, V.M.; Gänzle, M.G.; Schieber, A. Characterization of phenolic compounds in jocote (Spondias purpurea L.) peels by ultra high-performance liquid chromatography/electrospray ionization mass spectrometry. Food Res. Int. 2012, 46, 557–562. [Google Scholar] [CrossRef]
- Carvalho Gualberto, N.; Santos de Oliveira, C.; Pedreira Nogueira, J.; Silva de Jesus, M.; Caroline Santos Araujo, H.; Rajan, M.; Terezinha Santos Leite Neta, M.; Narain, N. Bioactive compounds and antioxidant activities in the agro-industrial residues of acerola (Malpighia emarginata L.), guava (Psidium guajava L.), genipap (Genipa americana L.) and umbu (Spondias tuberosa L.) fruits assisted by ultrasonic or shaker extraction. Food Res. Int. 2021, 147, 110538. [Google Scholar] [CrossRef]
- Servillo, L.; Giovane, A.; Balestrieri, M.L.; Ferrari, G.; Cautela, D.; Castaldo, D. Occurrence of Pipecolic Acid and Pipecolic Acid Betaine (Homostachydrine) in Citrus Genus Plants. J. Agric. Food Chem. 2012, 60, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; de Luca, V. The biosynthesis and regulation of biosynthesis of Concord grape fruit esters, including ‘foxy’ methylanthranilate. Plant J. 2005, 44, 606–619. [Google Scholar] [CrossRef]
- Semwal, R.; Joshi, S.K.; Semwal, R.B.; Semwal, D.K. Health benefits and limitations of rutin—A natural flavonoid with high nutraceutical value. Phytochem. Lett. 2021, 46, 119–128. [Google Scholar] [CrossRef]
- Costa, A.G.V.; Garcia-Diaz, D.F.; Jimenez, P.; Silva, P.I. Bioactive compounds and health benefits of exotic tropical red–black berries. J. Funct. Foods 2013, 5, 539–549. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Shrestha, S.; Lee, D.-Y.; Park, J.-H.; Cho, J.-G.; Seo, W.-D.; Kang, H.C.; Jeon, Y.-J.; Yeon, S.-W.; Bang, M.-H.; Baek, N.-I. Flavonoid glycosides from the fruit of Rhus parviflora and inhibition of cyclin dependent kinases by hyperin. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 689–693. [Google Scholar] [CrossRef]
- Kanchanapoom, T. Aromatic diglycosides from Cladogynos orientalis. Phytochemistry 2007, 68, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Rohde, A.; Morreel, K.; Ralph, J.; Goeminne, G.; Hostyn, V.; De Rycke, R.; Kushnir, S.; Van Doorsselaere, J.; Joseleau, J.-P.; Vuylsteke, M.; et al. Molecular Phenotyping of the pal1 and pal2 Mutants of Arabidopsis thaliana Reveals Far-Reaching Consequences on Phenylpropanoid, Amino Acid, and Carbohydrate Metabolism. Plant Cell 2004, 16, 2749–2771. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-Y.; Du, L.-D.; Lu, Y. Paeonol. In Natural Small Molecule Drugs from Plants; Springer: Singapore, 2018; pp. 439–444. ISBN 978-981-10-8022-7. [Google Scholar]
- Bohlinann, F.; Grenz, M. Neue Isopentenyl-acetophenon-Derivate aus Helianthella uniflora. Chem. Ber. 1970, 103, 90–96. [Google Scholar] [CrossRef]
- Tao, J.; Morikawa, T.; Ando, S.; Matsuda, H.; Yoshikawa, M. Bioactive Constituents from Chinese Natural Medicines. XI. Inhibitors on NO Production and Degranulation in RBL-2H3 from Rubia yunnanensis: Structures of Rubianosides II, III, and IV, Rubianol-g, and Rubianthraquinone. Chem. Pharm. Bull. 2003, 51, 654–662. [Google Scholar] [CrossRef] [Green Version]
- Lima, M.C.; Paiva de Sousa, C.; Fernandez-Prada, C.; Harel, J.; Dubreuil, J.D.; de Souza, E.L. A review of the current evidence of fruit phenolic compounds as potential antimicrobials against pathogenic bacteria. Microb. Pathog. 2019, 130, 259–270. [Google Scholar] [CrossRef]
- Dine Tariq Bouhlali, E.; Bammou, M.; Sellam, K.; Benlyas, M.; Alem, C.; Filali-Zegzouti, Y. Evaluation of antioxidant, antihemolytic and antibacterial potential of six Moroccan date fruit (Phoenix dactylifera L.) varieties. J. King Saud Univ.-Sci. 2016, 28, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Prasad, B.J.; Sharavanan, P.S.; Sivaraj, R. Efficiency of Oryza punctata extract on glucose regulation: Inhibition of α-amylase and α-glucosidase activities. Grain Oil Sci. Technol. 2019, 2, 44–48. [Google Scholar] [CrossRef]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef] [PubMed]
- Laaroussi, H.; Ferreira-Santos, P.; Genisheva, Z.; Bakour, M.; Ousaaid, D.; Teixeira, J.A.; Lyoussi, B. Unraveling the chemical composition, antioxidant, α-amylase and α-glucosidase inhibition of Moroccan propolis. Food Biosci. 2021, 42, 101160. [Google Scholar] [CrossRef]
- Jung, E.P.; Conrado Thomaz, G.F.; de Brito, M.O.; de Figueiredo, N.G.; Kunigami, C.N.; de Oliveira Ribeiro, L.; Alves Moreira, R.F. Thermal-assisted recovery of antioxidant compounds from Bauhinia forficata leaves: Effect of operational conditions. J. Appl. Res. Med. Aromat. Plants 2021, 22, 100303. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous optimization of several response variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid Determination of Polyphenols and Vitamin C in Plant-Derived Products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Gião, M.S.; González-Sanjosé, M.L.; Rivero-Pérez, M.D.; Pereira, C.I.; Pintado, M.E.; Malcata, F.X. Infusions of Portuguese medicinal plants: Dependence of final antioxidant capacity and phenol content on extraction features. J. Sci. Food Agric. 2007, 87, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Sánchez-Moreno, C.; de Pascual-Teresa, S. Flavonoid—Flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010, 121, 691–696. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 76, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Y.; Su, A.; Yuan, S.; Zhao, H.; Tan, S.; Hu, C.; Deng, H.; Guo, Y. Evaluation of Total Flavonoids, Myricetin, and Quercetin from Hovenia dulcis Thunb. As Inhibitors of α-Amylase and α-Glucosidase. Plant Foods Hum. Nutr. 2016, 71, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Statistica 13, version 13; Data analysis software system; Dell Inc.: Tulsa, OK, USA, 2015.



| Trials | Temperature | Ethanol | Solid–Liquid Ratio | TPC 1 | TFC 2 | ABTS•+ 3 | DPPH• 3 | FRAP 4 |
|---|---|---|---|---|---|---|---|---|
| (°C) | (%) | (g/mL) | ||||||
| 1 | 40 (−1) | 30 (−1) | 1:20 (−1) | 1280 | 925 | 74 | 95 | 319 |
| 2 | 40 (−1) | 30 (−1) | 1:50 (+1) | 1644 | 1015 | 83 | 136 | 364 |
| 3 | 40 (−1) | 80 (+1) | 1:20 (−1) | 603 | 692 | 25 | 49 | 150 |
| 4 | 40 (−1) | 80 (+1) | 1:50 (+1) | 811 | 700 | 25 | 82 | 130 |
| 5 | 65(+1) | 30 (−1) | 1:20 (−1) | 1593 | 1203 | 88 | 113 | 443 |
| 6 | 65 (+1) | 30 (−1) | 1:50 (+1) | 1677 | 1207 | 101 | 163 | 448 |
| 7 | 65 (+1) | 80 (+1) | 1:20 (−1) | 731 | 867 | 34 | 65 | 180 |
| 8 | 65 (+1) | 80 (+1) | 1:50 (+1) | 850 | 877 | 37 | 96 | 246 |
| 9 | 32 (−1.68) | 55 (0) | 1:35 (0) | 1231 | 847 | 58 | 105 | 289 |
| 10 | 74 (+1.68) | 55 (0) | 1:35 (0) | 1986 | 1513 | 109 | 162 | 504 |
| 11 | 53 (0) | 13 (−1.68) | 1:35 (0) | 1315 | 906 | 74 | 126 | 348 |
| 12 | 53 (0) | 97 (+1.68) | 1:35 (0) | 525 | 646 | 9 | 51 | 119 |
| 13 | 53 (0) | 55 (0) | 1:10 (−1.68) | 1075 | 1121 | 61 | 71 | 321 |
| 14 | 53 (0) | 55 (0) | 1:60 (+1.68) | 1652 | 1038 | 74 | 160 | 442 |
| 15 (CP) | 53 (0) | 55 (0) | 1:35 (0) | 1479 | 1055 | 73 | 121 | 316 |
| 16 (CP) | 53 (0) | 55 (0) | 1:35 (0) | 1379 | 1087 | 72 | 125 | 346 |
| 17 (CP) | 53 (0) | 55 (0) | 1:35 (0) | 1405 | 1186 | 77 | 128 | 364 |
| # | tR (min) | m/z Observed | m/z Theoretical | Molecular Formula | Fragment Ions (m/z) | Metabolite | Organism/Reference |
|---|---|---|---|---|---|---|---|
| 1 | 4.14 | 153.0200 | 153.0193 | C7H6O4 | 125.0261; 109.0279 | 3,5-Dihydroxybenzoic acid | Already described in Spondias spp. [19] |
| 2 | 18.45 | 609.1482 | 609.1461 | C27H30O16 | 301.0357; 300.0288; 273.0350; 257.0430; 151.0033 | Rutin | Already described in Spondias spp. [3,20] |
| 3 | 18.72 | 463.0862 | 463.0882 | C21H20O12 | 300.0256; 271.0236; 255.0342 | Isoquercitrin | Already described in Spondias spp. [19] |
| 4 | 20.62 | 593.1535 | 593.1512 | C27H30O15 | 285.0372; 284.0343; 257.0501; 255.0366; 227.0402 | Kaempferol 3-O-rutinoside | Already described in Spondias spp. [19] |
| 5 | 35.27 | 193.0709 | 193.0506 | C10H10O4 | 178.0512; 149.0979; 134.0676 | Ferulic acid | Already described in Spondias spp. [19] |
| # | tR (min) | m/z Observed | m/z Theoretical | Molecular Formula | Adduct | Fragment Ions (m/z) | Metabolite | Organism/Reference |
|---|---|---|---|---|---|---|---|---|
| 6 | 1.58 | 325.1329 | 325.1129 | C12H22O11 | [M − H2O + H]+ | 145.0502; 127.0399; 85.0297; 69.0342; 55.0188 | Sucrose | Very common in plants |
| 7 | 3.10 | 130.0863 | 130.0863 | C6H11NO2 | [M + H]+ | 84.0427; 57.0692; 56.0506 | Pipecolic acid | Found in Citrus spp. [21] |
| 8 | 7.47 | 165.0545 | 165.0546 | C9H8O3 | [M + H]+ | 147.0445; 120.0824; 119.0515 | Coumaric acid | Already described in Spondias spp. [3,20] |
| 9 | 7.82 | 347.1670 | 347.1337 | C15H22O9 | [M + H]+ | 185.0790; 154.0640; 153.0560; 125.0600 | 3,4,5-Trimethoxyphenyl beta-D-glucopyranoside (Koaburside) | Found in Rhus parviflora (Anacardiaceae) [26] |
| Found in Cladogynos orientalis (Euphorbiaceae) [27] | ||||||||
| 10 | 9.62 | 138.0557 | 138.0550 | C7H7NO2 | [M + H]+ | 121.0657; 92.9800; 65.0410 | Anthranilic acid | Found in Arabidopsis thaliana (Cruciferae) [28] |
| 2 | 18.40 | 611.1614 | 611.1607 | C27H30O16 | [M + H]+ | 465.1022; 303.0496; 145.0511; 129.0568 | Rutin | Already described in Spondias spp. [3,20] |
| 3 | 18.54 | 465.1028 | 465.1028 | C21H20O12 | [M + H]+ | 447.1002; 303.0463; 258.0178; 231.1018 | Isoquercitrin | Already described in Spondias spp. [19] |
| 11 | 19.42 | 167.0705 | 167.0703 | C9H10O3 | [M + H]+ | 149.0260; 125.0960; 121.0310 | 2’-Hydroxy-4’-methoxyacetophenone (Paeonol) | found in Paeonia spp. (Ranunculaceae) [29] |
| 12 | 36.01 | 205.1166 | 205.1223 | C13H16O2 | [M + H]+ | 149.0255; 121.0309; 107.0825; 59.0501 | 4-Acetyl-2-prenylphenol | Found in Polymnia sonchifolia (Asteraceae) [30] |
| 13 | 36.11 | 581.1551 | 581.1501 | C26H28O15 | [M + H]+ | 303.1460; 302.1490; 153.0967; 149.0236 | Quercetin-deoxyhexosyl-pentoside | Very common in plants |
| 14 | 38.09 | 389.2336 | 389.0843 | C17H18O9 | [M+Na]+ | 149.0240; 147.0656; 129.0550; 71.0850; 57.0705 | Rubinaphthin A | Found in Rubia spp. (Rubiaceae), i.e., Rubia yunnanensis [31] |
| 15 | 42.19 | 197.0812 | 197.0808 | C10H12O4 | [M + H]+ | 179.0861; 169.0027; 137.0633; 95.0850 | Dihydroferulic acid | Very common in plants |
| Microorganisms | Antimicrobial Assays (mg GAE/mL) ¹ | |
|---|---|---|
| MIC Values | MBC/MFC Values | |
| Gram-positive bacteria | ||
| Bacillus subtilis 168 LMD 74.6 | 0.06 | 0.12 |
| Staphylococcus aureus ATCC 29213 | 0.06 | 0.06 |
| Staphylococcus epidermidis ATCC 12228 | 0.03 | 0.12 |
| Gram-negative bacteria | ||
| Escherichia coli ATCC 25922 | 0.12 | 0.24 |
| Acinetobacter baumannii ATCC 19606 | 0.12 | 0.24 |
| Psedomonas aeruginosa ATCC 27853 | 0.12 | 0.24 |
| Klebsiella pneumoniae ATCC13883 | 0.12 | 0.24 |
| Fungi | ||
| Candida albicans ATCC 90028 | ND | ND |
| Candida tropicalis ATCC 750 | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, L.d.O.; de Freitas, B.P.; Lorentino, C.M.A.; Frota, H.F.; dos Santos, A.L.S.; Moreira, D.d.L.; do Amaral, B.S.; Jung, E.P.; Kunigami, C.N. Umbu Fruit Peel as Source of Antioxidant, Antimicrobial and α-Amylase Inhibitor Compounds. Molecules 2022, 27, 410. https://doi.org/10.3390/molecules27020410
Ribeiro LdO, de Freitas BP, Lorentino CMA, Frota HF, dos Santos ALS, Moreira DdL, do Amaral BS, Jung EP, Kunigami CN. Umbu Fruit Peel as Source of Antioxidant, Antimicrobial and α-Amylase Inhibitor Compounds. Molecules. 2022; 27(2):410. https://doi.org/10.3390/molecules27020410
Chicago/Turabian StyleRibeiro, Leilson de Oliveira, Beatriz Pereira de Freitas, Carolline Margot Albanez Lorentino, Heloisa Freire Frota, André Luis Souza dos Santos, Davyson de Lima Moreira, Bruno Sérgio do Amaral, Eliane Przytyk Jung, and Claudete Norie Kunigami. 2022. "Umbu Fruit Peel as Source of Antioxidant, Antimicrobial and α-Amylase Inhibitor Compounds" Molecules 27, no. 2: 410. https://doi.org/10.3390/molecules27020410
APA StyleRibeiro, L. d. O., de Freitas, B. P., Lorentino, C. M. A., Frota, H. F., dos Santos, A. L. S., Moreira, D. d. L., do Amaral, B. S., Jung, E. P., & Kunigami, C. N. (2022). Umbu Fruit Peel as Source of Antioxidant, Antimicrobial and α-Amylase Inhibitor Compounds. Molecules, 27(2), 410. https://doi.org/10.3390/molecules27020410