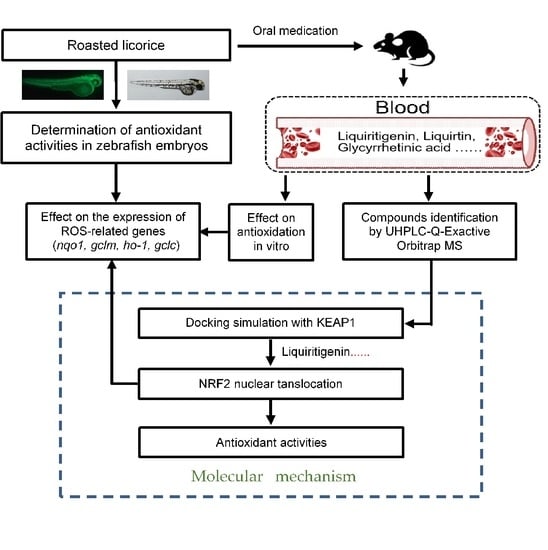
Antioxidant Effects of Roasted Licorice in a Zebrafish Model and Its Mechanisms
Abstract
:1. Introduction
2. Results
2.1. Roasted Licorice Extracts Protect against Oxidative Stress Injury and Excessive ROS Generation In Vivo
2.2. The Antioxidant Activity of Roasted Licorice Extracts Is Associated with the Transcriptional Level of Oxidant Stress-Related Genes In Vivo
2.3. The Antioxidant Activity of Roasted Licorice Extracts Is Associated with the Transcriptional Level of Oxidant Stress-Related Genes In Vitro
2.4. Phytochemical Analysis of Roasted Licorice Extracts
2.5. The Major Phytochemicals in Roasted Licorice Extracts Show Potential Binding Activity with Keap1 according to a Molecular Docking Simulation
2.6. Liquiritigenin Promotes the Nuclear Translocation of NRF2
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Cells and Culture Conditions
4.4. Sample Preparation
4.4.1. Roasted Licorice
4.4.2. Roasted Licorice Extracts for Zebrafish Model Experiment
4.4.3. Plasma Samples for UHPLC–Q-Exactive Orbitrap MS Detection
4.4.4. Serum Samples for Cell Experiment
4.5. Establishment of MTZ-Induced Oxidative Stress Model In Vivo
4.6. Establishment of H2O2-Induced Oxidative Stress Model In Vitro
4.7. Detection of Antioxidant Activities In Vivo
4.8. Quantitative Real-Time PCR (qRT-PCR)
4.9. Apoptosis Detection
4.10. Identification of Compounds Using UHPLC–Q-Exactive Orbitrap MS
4.11. Molecular Docking in Silico
4.12. Immunofluorescence
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majima, T.; Yamada, T.; Tega, E.; Sakurai, H.; Saiki, I.; Tani, T. Pharmaceutical evaluation of liquorice before and after roasting in mice. J. Pharm. Pharmacol. 2004, 56, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.K.; Lim, S.S.; Choi, K.H.; Yoo, K.Y.; Shin, H.K.; Kim, E.J.; Yoon-Park, J.H.; Kang, T.C.; Kim, Y.S.; Kwon, D.Y.; et al. Neuroprotective effects of roasted licorice, not raw form, on neuronal injury in gerbil hippocampus after transient forebrain ischemia. Acta Pharmacol. Sin. 2006, 27, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Xu, W. Research on Antioxidant Ability of Licorice Extracts and the Application in Rabbit Meat. Master’s Thesis, Southwest University, Chongqing, China, 2017. [Google Scholar]
- Cao, S.Y. Development of Functional Cosmetics Based on the Natural Drug-Glycyrrhiza Active Component. Master’s Thesis, Changchun University of Chinese Medicine, Changchun, China, 2019. [Google Scholar]
- Quintana, S.E.; Cueva, C.; Villanueva-Bermejo, D.; Moreno-Arribas, M.V.; Fornari, T.; García-Risco, M.R. Antioxidant and antimicrobial assessment of licorice supercritical extracts. Ind. Crops Prod. 2019, 139, 111496. [Google Scholar] [CrossRef]
- Reigada, I.; Moliner, C.; Valero, M.S.; Weinkove, D.; Langa, E.; Gómez Rincón, C. Antioxidant and Antiaging Effects of Licorice on the Caenorhabditis elegans Model. J. Med. Food 2020, 23, 72–78. [Google Scholar] [CrossRef]
- Zhang, F.X.; Song, J.X.; Liu, X.D.; Shan, H. Antioxidation and immune activity of licorice flavonoids. Chin. J. Vet. Sci. 2019, 39, 1180–1183. [Google Scholar]
- Zhang, C.H.; Yu, Y.; Liang, Y.Z.; Chen, X.Q. Purification, partial characterization and antioxidant activity of polysaccharides from Glycyrrhiza uralensis. Int. J. Biol. Macromol. 2015, 79, 681–686. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, W.B. Study on chemical constituents and pharmacological action of Licorice. Hunan Feed 2017, 21–23. [Google Scholar]
- Zhao, F.F. The Effect of Glycyrrhiza Uralensis Fisch on D-Gal Induced Aging Rats and the Analysis of the Target Metabolomics Based on Taurine Pathway. Master’s Thesis, Shanxi University, Taiyuan, China, 2018. [Google Scholar]
- Li, S. The Effect and Mechanism of Glycyrrhizic acid on Human Glioma U251 Cells. Master’s Thesis, Southern Medical University, Guangzhou, China, 2014. [Google Scholar]
- Chen, X.; Liu, Z.; Meng, R.; Shi, C.; Guo, N. Antioxidative and anticancer properties of Licochalcone A from licorice. J. Ethnopharmacol. 2017, 198, 331–337. [Google Scholar] [CrossRef]
- Chandrasekaran, C.V.; Deepak, H.B.; Thiyagarajan, P.; Kathiresan, S.; Sangli, G.K.; Deepak, M.; Agarwal, A. Dual inhibitory effect of Glycyrrhiza glabra (GutGard) on COX and LOX products. Phytomedicine 2011, 18, 278–284. [Google Scholar] [CrossRef]
- Kim, J.K.; Oh, S.M.; Kwon, H.S.; Oh, Y.S.; Lim, S.S.; Shin, H.K. Anti-inflammatory effect of roasted licorice extracts on lipopolysaccharide-induced inflammatory responses in murine macrophages. Biochem. Biophys. Res. Commun. 2006, 345, 1215–1223. [Google Scholar] [CrossRef]
- Ota, M.; Nagachi, Y.; Ishiuchi, K.; Tabuchi, Y.; Xu, F.; Shang, M.Y.; Cai, S.Q.; Makino, T. Comparison of the inducible effects of licorice products with or without heat-processing and pre-treatment with honey on granulocyte colony-stimulating factor secretion in cultured enterocytes. J. Ethnopharmacol. 2018, 214, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Afanas’ev, I. Signaling and Damaging Functions of Free Radicals in Aging―Free Radical Theory, Hormesis, and TOR. Aging Dis. 2010, 1, 75–88. [Google Scholar] [PubMed]
- Kulkarni, A.A.; Conteh, A.M.; Sorrell, C.A.; Mirmira, A.; Tersey, S.A.; Mirmira, R.G.; Linnemann, A.K.; Anderson, R.M. An in Vivo Zebrafish Model for Interrogating ROS-Mediated Pancreatic beta-Cell Injury, Response, and Prevention. Oxid. Med. Cell Longev. 2018, 2018, 1324739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.A.; Lee, S.H.; Ko, C.I.; Cha, S.H.; Kang, M.C.; Kang, S.M.; Ko, S.C.; Lee, W.W.; Ko, J.Y.; Lee, J.H.; et al. Protective effect of fucoidan against AAPH-induced oxidative stress in zebrafish model. Carbohydr. Polym. 2014, 102, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Dong, J.; Wang, H.; Wang, L.R.; Duan, Y.S.; Chen, S.Z. Fragmentation Study of the C Ring in Flavone and Isoflavone Aglycones by Electrospray Ion Trap Time-of-Flight Mass Spectrometry. Chem. J. Chin. Univ. 2009, 30, 46–50. [Google Scholar]
- Zhang, Y.; Yang, A.D.; Gao, J. Identification of main chemical constituents in extracts of Glycyrrhizae Radix et Rhizoma by HPLC/ESI-MS. Chin. Tradit. Pat. Med. 2012, 34, 1111–1115. [Google Scholar]
- Yu, X.M.; Wang, H.C.; Tian, L.L.; Meng, X.S.; Ying, X.X. Preliminary study on serum pharmacochemistry of Qizhiweitong Particles based on gastrointestinal motility disorder in rats. J. Pharm. Res. 2017, 36, 63–66. [Google Scholar] [CrossRef]
- Li, A.L.; Shen, T.; Wang, T.; Zhou, M.X.; Wang, B.; Song, J.T.; Zhang, P.L.; Wang, X.L.; Ren, D.M.; Lou, H.X.; et al. Novel diterpenoid-type activators of the Keap1/Nrf2/ARE signaling pathway and their regulation of redox homeostasis. Free Radic. Biol. Med. 2019, 141, 21–33. [Google Scholar] [CrossRef]
- Zhang, L.P.; Zheng, Q. Application Analysis of Radix Glycyrrhizae and Baked Radix Glycyrrhizae in Classical Prescriptions. J. Basic Chin. Med. 2022, 28, 1495–1498. [Google Scholar] [CrossRef]
- Luo, L.P.; Li, Y.J.; Zhang, H.; Li, P.; Chen, J. Determination of antioxidant activity in licorice vitro metabolites by DPPH spiking coupled with HPLC-Q-TOF MS/MS. Chin. J. New Drugs 2013, 22, 2547–2552. [Google Scholar]
- Ji, W.; Li, C.D.; Li, Z.Y.; Sun, J.J.; Fu, Y.J. Effect of isoliquiritigenin on LDH and SOD activity in PC12 cells before and after hypoxia. Chin. J. Neurosurg. Dis. Res. 2016, 15, 132–135. [Google Scholar]










| Peak | Rt/min | Molecular Formula | Compound | Ion Mode | m/z | ppm | Secondary Fragment |
|---|---|---|---|---|---|---|---|
| 1 | 0.85 | C5H9NO2 | l-Proline | [M+H]+ | 116.0708 | 1.335 | 116.0708, 70.0657, 68.0601 |
| 2 | 7.8 | C26H30O13 | Liquiritin apioside | [M−H]− | 549.1615 | 2.135 | 549.1615, 255.0661, 153.0162, 135.0075, 119.0490 |
| 3 | 7.81 | C21H21O10 | Glucuronides of liquirtigenin | [M−H]− | 431.0999 | 2.607 | 431.0999, 255.0663, 153.0184, 135.0077, 119.0491 |
| 4 | 7.85 | C21H22O9 | Liquiritin | [M−H]− | 417.1191 | 2.568 | 417.1191, 255.0665, 153.0184, 135.0078, 119.0492 |
| 5 | 8.2 | C15H1107S | Sulfates of liquirtigenin | [M−H]− | 335.0233 | 3.941 | 335.0233, 255.0661, 153.0183, 135.0076, 119.0490 |
| 6 | 8.56 | C21H21O10 | Glucuronides of isoliquirtigenin | [M+H]+ | 433.1101 | −6.426 | 433.1101, 257.0803, 239.0692, 147.0438, 137.0232 |
| 7 | 8.63 | C22H22O9 | Ononin | [M+H]+ | 431.1331 | −1.18 | 431.1331, 269.0804, 254.0570, 213.0905, 197.0594, 137.0233, 118.0413 |
| 8 | 8.64 | C15H12O4 | Liquiritigenin | [M−H]− | 255.0654 | 0.842 | 255.0654, 153.0182, 149.0233, 135.0076, 119.0490 |
| 9 | 9.05 | C16H14O4 | Echinatin | [M+H]+ | 271.0956 | −3.451 | 271.0956, 256.0713, 161.0594, 137.0596, 123.0441 |
| 10 | 9.06 | C15H1107S | Sulfates of isoliquirtigenin | [M−H]− | 335.0232 | 3.553 | 335.0232, 255.0660, 153.0182, 135.0076, 119.0490 |
| 11 | 11.28 | C20H18O6 | Licoflavonol | [M−H]− | 353.1029 | 2.762 | 353.1029, 243.1028, 201.0919, 83.0123 |
| 12 | 11.32 | C36H53O10 | Glucuronides of glycyrrhetinic scid | [M+H]+ | 647.3789 | −0.099 | 647.3789, 471.3461, 407.3277, 317.2097, 235.1688 |
| [M−H]− | 645.3643 | 1.512 | 645.3643, 469.3322, 425.3436 | ||||
| 13 | 12.3 | C20H16O6 | Semilicoisoflavone B | [M−H]− | 351.0873 | 2.750 | 351.0873, 307.0961, 283.0977, 265.0872, 241.0864, 199.0758, 83.0124 |
| 14 | 14.42 | C30H46O4 | Glycyrrhetinic acid | [M+H]+ | 471.3464 | −1.096 | 471.3464, 453.3372, 407.3294, 317.2109, 271.2059, 235.1690, 189.1636 |
| [M−H]− | 469.3321 | 1.883 | 469.3321, 425.3422, 355.2634, 161.3441 | ||||
| 15 | 15.55 | C30H44O4 | Glabrolide | [M−H]− | 467.3167 | 2.405 | 467.3167, 423.3258, 407.2991, 353.2492 |
| Receptors | -CDocker Energy (kcal/mol) | -CDocker Interaction Energy (kcal/mol) | |
|---|---|---|---|
| Ligands | |||
| Echinatin | 25.4205 | 42.3573 | |
| Glabrolide | −43.6623 | 59.5078 | |
| Glycyrrhetinic acid | −24.6014 | 64.0249 | |
| Licoflavonol | 25.0266 | 51.83 | |
| Liquiritigenin | 36.1318 | 40.8838 | |
| Liquiritin apioside | 7.21328 | 75.059 | |
| Liquiritin | 29.3572 | 58.8813 | |
| l-Proline | 4.2125 | 21.246 | |
| Ononin | 23.0989 | 63.571 | |
| Semilicoisoflavone B | 31.6962 | 48.8378 | |
| Genes | Forward | Reverse | |
|---|---|---|---|
| Zebrafish | ho-1 | 5′–AAGCAAAGCGGCAGAGAAC–3′ | 5′–TGGAGCAGTCAGATGAAGTGT–3′ |
| nqo1 | 5′–CTGGGTGGTGTGTTTGAAGAA–3′ | 5′–GCTGTGGTAATGCCGTAGG–3′ | |
| gclm | 5′–TCGCTCCTCCTTCTCTTCC–3′ | 5′–CCGATGGCAGCAATCTTCT–3′ | |
| gclc | 5′–CGGATGGAGAGTGGAGTTCA–3′ | 5′–TTCGCTTCTGGGCTACCTT–3′ | |
| Human | HO-1 | 5′–AAGACTGCGTTCCTGCTCAAC–3′ | 5′–AAAGCCCTACAGCAACTGTCG–3′ |
| NQO1 | 5′–GAAGAGCACTGATCGTACTGGC–3′ | 5′–GGATACTGAAAGTTCGCAGGG–3′ | |
| GCLM | 5′–CATTTACAGCCTTACTGGGAGG–3′ | 5′–ATGCAGTCAAATCTGGTGGCA–3′ | |
| GCLC | 5′–GGCACAAGGACGTTCTCAAGT–3′ | 5′–CAGACAGGACCAACCGGAC–3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Zhang, S.; Geng, X.; Jiang, H.; Dai, Y.; Wang, P.; Hua, M.; Gao, Q.; Lang, S.; Hou, L.; et al. Antioxidant Effects of Roasted Licorice in a Zebrafish Model and Its Mechanisms. Molecules 2022, 27, 7743. https://doi.org/10.3390/molecules27227743
Zhou Q, Zhang S, Geng X, Jiang H, Dai Y, Wang P, Hua M, Gao Q, Lang S, Hou L, et al. Antioxidant Effects of Roasted Licorice in a Zebrafish Model and Its Mechanisms. Molecules. 2022; 27(22):7743. https://doi.org/10.3390/molecules27227743
Chicago/Turabian StyleZhou, Qian, Shanshan Zhang, Xue Geng, Haiqiang Jiang, Yanpeng Dai, Ping Wang, Min Hua, Qi Gao, Shiyue Lang, Lijing Hou, and et al. 2022. "Antioxidant Effects of Roasted Licorice in a Zebrafish Model and Its Mechanisms" Molecules 27, no. 22: 7743. https://doi.org/10.3390/molecules27227743
APA StyleZhou, Q., Zhang, S., Geng, X., Jiang, H., Dai, Y., Wang, P., Hua, M., Gao, Q., Lang, S., Hou, L., Shi, D., & Zhou, M. (2022). Antioxidant Effects of Roasted Licorice in a Zebrafish Model and Its Mechanisms. Molecules, 27(22), 7743. https://doi.org/10.3390/molecules27227743