Evaluation of Cosmetic and Dermatological Properties of Kombucha-Fermented Berry Leaf Extracts Considered to Be By-Products
Abstract
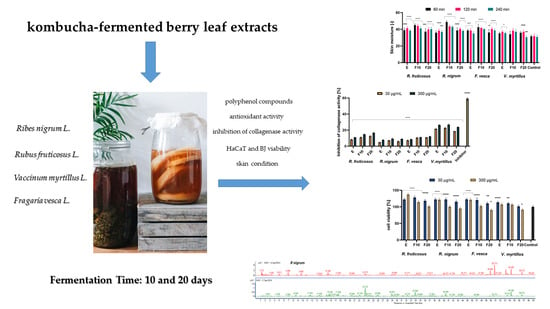
:1. Introduction
2. Results and Discussion
2.1. Determination of Bioactive Compounds
2.2. Assessment of Antioxidant Activity
2.3. Cytotoxicity Assessment
2.4. Assessment of Matrix Metallopeptidades Inhibition
2.5. Influence of the Extracts and Ferments on the Skin Condition
3. Materials and Methods
3.1. Plant Material and Fermentation Procedure
3.2. Determination of Biologically Active Compounds
3.3. Assessment of Antioxidant Activity
3.3.1. DPPH Radical Scavenging Assay
3.3.2. ABTS+ Scavenging Assay
3.3.3. Determination of Intracellular Levels of Reactive Oxygen Species (ROS)
3.4. Cytotoxicity Analysis
3.4.1. Cell Culture
3.4.2. Alamar Blue Assay
3.4.3. Neutral Red Uptake Assay
3.5. Assessment of Matrix Metallopeptidades Inhibition
3.5.1. Determination of Anti-Collagenase Activity
3.5.2. Determination of Anti-Elastase Activity
3.6. Measurement of Transepidermal Water Loss (TEWL), Skin Hydration and Skin pH
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ferreira, M.S.; Magalhães, M.C.; Oliveira, R.; Sousa-Lobo, J.M.; Almeida, I.F. Trends in the Use of Botanicals in Anti-Aging Cosmetics. Molecules 2021, 26, 3584. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Jerold, F. Biocosmetics: Technological advances and future outlook. Environ. Sci. Pollut. Res. Int. 2021, 25, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Faccio, G. Plant Complexity and Cosmetic Innovation. iScience 2020, 23, 101358. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, K.G.O.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Saponins and microbial biosurfactants: Potential raw materials for the formulation of cosmetics. Biotechnol. Prog. 2018, 34, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Piazza, S.; Fumagalli, M.; Khalilpour, S.; Martinelli, G.; Magnavacca, A.; Dell’Agli, M.; Sangiovanni, E. A Review of the Potential Benefits of Plants Producing Berries in Skin Disorders. Antioxidants 2020, 9, 542. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z. Assessment of cytotoxicity and antioxidant properties of berry leaves as by-products with potential application in cosmetic and pharmaceutical products. Sci. Rep. 2021, 11, 3240. [Google Scholar] [CrossRef] [PubMed]
- Cádiz-Gurrea, M.L.; Pinto, D.; Delerue-Matos, C.; Rodrigues, F. Olive Fruit and Leaf Wastes as Bioactive Ingredients for Cosmetics-A Preliminary Study. Antioxidants 2021, 10, 245. [Google Scholar] [CrossRef]
- Paredes-López, O.; Cervantes-Ceja, M.L.; Vigna-Pérez, M.; Hernández-Pérez, T. Berries: Improving human health and healthy aging, and promoting quality life—A review. Plant Foods Hum. Nutr. 2010, 65, 299–308. [Google Scholar] [CrossRef]
- Ferlemi, A.V.; Lamari, F.N. Berry Leaves: An Alternative Source of Bioactive Natural Products of Nutritional and Medicinal Value. Antioxidants 2016, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Cyboran, S.; Bonarska-Kujawa, D.; Pruchnik, H.; Żyłka, R.; Oszmiański, J.; Kleszczyńska, H. Phenolic content and biological activity of extracts of blackcurrant fruit and leaves. Food Res. Int. 2010, 65, 47–58. [Google Scholar] [CrossRef]
- Tabart, J.; Kevers, C.; Evers, D.; Dommes, J. Ascorbic acid, phenolic acid, flavonoid, and carotenoid profiles of selected extracts from Ribes nigrum. J. Agric. Food Chem. 2011, 59, 4763–4770. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-T.; Sim, G.-S.; Kim, J.-H.; Pyo, H.-B.; Yun, J.-W.; Lee, B.-C. Antioxidative activity of the hydrolytic enzyme treated Sorbus commixta Hedl. and its inhibitory effect on matrix metalloproteinase-1 in UV irradiated human dermal fibroblasts. Arch. Pharm. Res. 2007, 30, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Warraicha, U.E.; Hussaina, F.; Kayanib, H.U.R. Aging-Oxidative stress, antioxidants and computational modeling. Heliyon 2020, 6, e04107. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharmacol. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, Y.Z.; Wang, M. Bioactive substances of plant origin. In Handbook of Food Chemistry, 1st ed.; Cheung, P., Mehta, B., Eds.; Springer: Heidelberg, Germany, 2015; pp. 967–1008. [Google Scholar]
- Sadh, P.K.; Kumar, S.; Chawla, P.; Duhan, J.S. Fermentation: A Boon for Production of Bioactive Compounds by Processing of Food Industries Wastes (By-Products). Molecules 2018, 23, 2560. [Google Scholar] [CrossRef] [Green Version]
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef]
- Antolak, H.; Piechota, D.; Kucharska, A. Kombucha Tea—A Double Power of Bioactive Compounds from Tea and Symbiotic Culture of Bacteria and Yeasts (SCOBY). Antioxidants 2021, 10, 1541. [Google Scholar] [CrossRef]
- Emiljanowicz, K.E.; Malinowska-Pańczyk, E. Kombucha from alternative raw materials—The review. Crit. Rev. Food Sci. Nutr. 2019, 60, 3185–3194. [Google Scholar] [CrossRef]
- Xia, X.; Dai, Y.; Wu, H.; Liu, X.; Wang, Y.; Yin, L.; Zhou, J. Kombucha fermentation enhances the health-promoting properties of soymilk beverage. J. Funct. Foods 2019, 62, 103549. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Ziemlewska, A.; Bujak, T.; Zagórska-Dziok, M.; Zarębska, M.; Hordyjewicz-Baran, Z.; Wasilewski, T. Effect of Fermentation Time on Antioxidant and Anti-Ageing Properties of Green Coffee Kombucha Ferments. Molecules 2020, 25, 5394. [Google Scholar] [CrossRef]
- Pure, A.E.; Pure, M.E. Antioxidant and antibacterial activity of kombucha beverages prepared using banana peel, common nettles and black tea infusions. Appl. Food Biotechnol. 2016, 3, 125–130. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Bujak, T.; Zagórska-Dziok, M.; Wójciak, M.; Sowa, I. Effect of fermentation time on the content of bioactive compounds with cosmetic and dermatological properties in Kombucha Yerba Mate extracts. Sci. Rep. 2021, 11, 18792. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wołdyło, A.; Nowicka, P.; Teleszko, M.; Cebulak, T.; Wolanin, M. Determination of phenolic compounds and antioxidant activity in leaves from wild Rubus L. species. Molecules 2015, 20, 4951–4966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bujor, O.; Le, B.C.; Volf, I.; Popa, V.I.; Dufour, C. Seasonal variations of the phenolic constituents in bilberry (Vaccinium myrtillus L.) leaves, stems and fruits, and their antioxidant activity. Food Chem. 2016, 213, 58–68. [Google Scholar] [CrossRef]
- D’Urso, G.; Montoro, P.; Piacente, S. Detection and comparison of phenolic compounds in different extracts of black currant leaves by liquid chromatography coupled with high-resolution ESI-LTQ-Orbitrap MS and high-sensitivity ESI-Qtrap MS. J. Pharm. Biomed. Anal. 2020, 179, 112926. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, G.; Pizza, C.; Piacente, S.; Montoro, P. Combination of LC-MS based metabolomics and antioxidant activity for evaluation of bioactive compounds in Fragaria vesca leaves from Italy. J. Pharm. Biomed. Anal. 2018, 150, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Čanadanović-Brunet, J.M. Infuence of starter cultures on the antioxidant activity of Kombucha beverage. Food Chem. 2011, 127, 1727–1731. [Google Scholar] [CrossRef]
- Ivanišová, E.; Meňhartová, K.; Terentjeva, M.; Godočíková, L.; Árvay, J.; Kačániová, M. Kombucha tea beverage: Microbiological characteristic, antioxidant activity, and phytochemical composition. Acta Aliment. 2019, 48, 324–331. [Google Scholar] [CrossRef]
- Jayabalan, R.; Subathradevi, P.; Marimuthu, S.; Sathishkumar, M.; Swaminathan, K. Changes in free-radical scavenging ability of kombucha tea during fermentation. Food Chem. 2008, 109, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Sagar, B.; Kedare, R.P. Singh: Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Chanput, W.; Krueyos, N.; Ritthiruangdej, P. Anti-oxidative assays as markers for anti-inflammatory activity of flavonoids. Int. Immunopharmacol. 2016, 40, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Teleszko, M.; Wojdyło, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods. 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Rosa, A.; Bifulco, E.; Melis, M.P.; Atzeri, A.; Pirisi, F.M.; Dessi, M.A. Chemical composition and antioxidant activities of Myrtus communis L. berries extracts. Food Chem. 2010, 123, 1242–1251. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Gachhui, R.; Sil, P.C. Effect of kombucha, a fermented black tea in attenuating oxidative stress mediated tissue damage in alloxan-induced diabetic rats. Food Chem. Toxicol. 2013, 60, 328–340. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell Longev. 2016, 12, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Ahn, C.B.; Je, J.Y. Antioxidant activity of traditional Korean fermented soybean (DAMDUSI) extract on free radical-mediated oxidative systems. J. Food Biochem. 2011, 35, 1242–1256. [Google Scholar] [CrossRef]
- Hu, C.-C.; Hsiao, C.-H.; Huang, S.-Y.; Fu, S.-H.; Lai, C.-C.; Hong, T.-M.; Lu, F.-J. Antioxidant activity of fermented soybean extract. J. Agric. Food Chem. 2004, 52, 5735–5739. [Google Scholar] [CrossRef]
- Pyo, Y.-H.; Lee, T.-C. The potential antioxidant capacity and angiotensin I-converting enzyme inhibitory activity of Monascus-fermented soybean extracts: Evaluation of Monascus-fermented soybean extracts as multifunctional food additives. J. Food Sci. 2007, 72, S218–S223. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.K.; Appaiah, K.A. Application of native yeast from Garcinia (Garcinia xanthochumus) for the preparation of fermented beverage: Changes in biochemical and antioxidant properties. Food Biosci. 2014, 5, 101–107. [Google Scholar] [CrossRef]
- Kim, J.-H.; Baik, S.-H. Preparation and characterization of fermented dandelion (Taraxacum officinale) beverage using Lactobacillus acidophilus F46 having cinnamoyl esterase activity. Food Sci. Biotechnol. 2015, 24, 583–593. [Google Scholar] [CrossRef]
- Milivojevic, J.; Maksimovic, V.; Nikolic, M.; Bogdanovic, J.; Maletic, R.; Milatovic, D. Chemical and antioxidant properties of cultivated and wild Fragaria and Rubus berries. J. Food Qual. 2011, 34, 1–9. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Riaz, M.; De Feo, V.; Jaafar, H.Z.; Moga, M. Rubus fruticosus L.: Constituents, biological activities and health related uses. Molecules 2014, 19, 10998–11029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalan, A.; Reuben, S.C.; Ahmed, S.; Darvesh, A.S.; Hohmann, J.; Bishayee, A. The health benefits of blackcurrants. Food Funct. 2012, 3, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Leyden, J.J.; Rewlings, A.V. Skin Moisturization, 1st ed.; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Olas, B. Berry Phenolic Antioxidants—Implications for Human Health? Front. Pharmacol. 2018, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Ribera-Fonseca, A.; Jiménez, D.; Leal, P.; Riquelme, I.; Roa, J.C.; Alberdi, M.; Peek, R.M.; Reyes-Díaz, M. The anti-proliferative and anti-invasive effect of leaf extracts of blueberry plants treated with methyl jasmonate on human gastric cancer in vitro is related to their antioxidant properties. Antioxidants 2020, 4, 45. [Google Scholar] [CrossRef] [Green Version]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on Kombucha Tea-Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Cetojevic-Simin, D.D.; Bogdanovic, G.M.; Cvetkovic, D.D.; Velicanski, A.S. Antiproliferative and antimicrobial activity of traditional Kombucha and Satureja montana L. Kombucha. J. BUON 2008, 13, 395–401. [Google Scholar] [PubMed]
- DEGHRIGUE, M.; Chriaa, J.; Battikh, H.; Kawther, A.B.I.D.; Bakhrouf, A. Antiproliferative and antimicrobial activities of kombucha tea. Afr. J. Microbiol. Res. 2013, 7, 3466–3470. [Google Scholar] [CrossRef]
- Ďuračková, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Martino, E.; Ilisso, C.P.; Bagarolo, M.L.; Porcelli, M.; Cacciapuoti, G. Pro-oxidant and pro-apoptotic activity of polyphenol extract from Annurca apple and its underlying mechanisms in human breast cancer cells. Int. J. Oncol. 2017, 51, 939–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Madan, K.; Nanda, S. In-vitro evaluation of antioxidant, anti-elastase, anti-collagenase, antihyaluronidase activities of safranal and determination of its sun protection factor in skin photoaging. Bioorganic Chem. 2018, 77, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiratchayamaethasakul, C.; Ding, Y.; Hwang, O.; Im, S.T.; Jang, Y.; Myung, S.W. In vitro screening of elastase, collagenase, hyaluronidase, and tyrosinase inhibitory and antioxidant activities of 22 halophyte plant extracts for novel cosmeceuticals. Fish. Aquat. Sci. 2020, 23, 6. [Google Scholar] [CrossRef]
- Choi, M.-H.; Kim, Y.-W.; Kim, M.-S.; Shin, H.-J. Development of Cosmetic Emulsion Using Blueberry Fruit Extract and Agarose from Gracilaria verrucosa. KSBB J. Korean Soc. Biotechnol. Bioeng. 2016, 31, 256–262. [Google Scholar]
- Lukitaningsih, E.; Nur, S.; Qonithah, F.; Zulbayu, M.; Kuswahyuning, R.; Rumiyati, R. In Vitro Anti-Wrinkle and Tyrosinase Inhibitory Activities of Grapefruit Peel and Strawberry Extracts. Maj. Obat Tradis. 2020, 25, 182–189. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Leopold, L.; Rugină, D.; Ayvaz, H.; Socaciu, C. Antiproliferative and Antioxidant Properties of Anthocyanin Rich Extracts from Blueberry and Blackcurrant Juice. Int. J. Mol. Sci. 2015, 16, 2352–2365. [Google Scholar] [CrossRef] [Green Version]
- Bonté, F. Skin moisturization mechanisms: New data. Ann. Pharm. Françaises 2011, 69, 135–141. [Google Scholar] [CrossRef]
- Wang, G.-H.; Chen, C.-Y.; Tsai, T.-H.; Chen, C.-K.; Cheng, C.-Y.; Huang, Y.-H.; Hsieh, M.-C.; Chung, Y.-C. Evaluation of tyrosinase inhibitory and antioxidant activities of Angelica dahurica root extracts for four different probiotic bacteria fermentations. J. Biosci. Bioeng. 2017, 123, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Petruk, G.; DelGiudice, R.; Rigano, M.M.; Monti, D.M. Antioxidants from Plants Protect against Skin Photoaging. Oxid. Med. Cell. Longev. 2018, 2018, 1454936. [Google Scholar] [CrossRef] [Green Version]
- Malbaša, R.V.; Lončar, E.S.; Kolarov, L.J.A. Sucrose and inulin balance during tea fungus fermentation. Roum. Biotechnol. Lett. 2002, 7, 573–576. [Google Scholar]
- Chen, C.; Liu, B.Y. Changes in major components of tea fungus metabolites during prolonged fermentation. J. Appl. Microbiol. 2000, 89, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Saba, A.M.; Yosipovitch, G. Skin pH: From basic science to basic skin care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Schmid-Wendtner, M.H.; Korting, H.C. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.L.; Villarreal, M.; Jepson, B.; Rafaels, N.; David, G.; Hanifin, J.; Beck, L.A. Patients with atopic dermatitis colonized with Staphylococcus aureus have a distinct phenotype and endotype. J. Investig. Dermatol. 2018, 138, 2224–2233. [Google Scholar] [CrossRef] [Green Version]
- Walters, R.M.; Mao, G.; Gunn, E.T.; Hornby, S. Cleansing formulations that respect skin barrier integrity. Dermatol. Res. Pract. 2012, 2012, 495917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, R.; Parish, L.C. Effect of soaps and detergents on epidermal barrier function. Clin. Dermatol. 2012, 30, 297–300. [Google Scholar] [CrossRef]
- Korting, H.C.; Kober, M.; Müller, M.; Braun-Falco, O. Influence of repeated washings with soap and synthetic detergents on pH and resident flora of the skin of forehead and forearm. Acta Derm. Venereol. 1987, 67, 41–47. [Google Scholar] [PubMed]
- Korting, H.C.; Hübner, K.; Greiner, K.; Hamm, G.; Braun-Falco, O. Differences in the skin surface pH and bacterial microflora due to the long-term application of synthetic detergent preparations of pH 5.5 and pH 7.0. Acta Derm. Venereol. 1990, 70, 429–457. [Google Scholar] [PubMed]
- Yang, B.; Liu, X.; Gao, Y. Extraction optimization of bioactive compounds crocin, geniposide and total phenolic compounds from Gardenia Gardenia jasminoides Ellis fruits with response surface methodology. Innov. Food Sci. Emerg. Technol. 2009, 10, 610–615. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Gaweł-Beben, K.; Bujak, T.; Nizioł-Łukaszewska, Z.; Antosiewicz, B.; Jakubczyk, A.; Karas, M.; Rybczynska, K. Stevia Rebaudiana Bert. leaf extracts as a multifunctional source of natural antioxidants. Molecules 2015, 20, 5468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evangelista-Vargas, S.; Santiani, A. Detection of intracellular reactive oxygen species (superoxide anion and hydrogen peroxide) and lipid peroxidation during cryopreservation of alpaca spermatozoa. Reprod. Domest. Anim. 2017, 52, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Nizioł-Łukaszewska, Z.; Zagórska-Dziok, M.; Ziemlewska, A.; Bujak, T. Comparison of the antiaging and protective properties of plants from the Apiaceae family. Oxid. Med. Cell. Longev. 2020, 2020, 5307614. [Google Scholar] [CrossRef]
- Mertz, C.; Cheynier, V.; Günata, Z.; Brat, P. Analysis of Phenolic Compounds in Two Blackberry Species (Rubus glaucus and Rubus adenotrichus) by High-Performance Liquid Chromatography with Diode Array Detection and Electrospray Ion Trap Mass Spectrometry. J. Agric. Food Chem. 2007, 55, 8616–8624. [Google Scholar] [CrossRef]
- Oszmianski, J.; Wojdyło, A.; Gorzelany, J.; Kapusta, I. Identification and Characterization of Low Molecular Weight Polyphenols in Berry Leaf Extracts by HPLC-DAD and LC-ESI/MS. J. Agric. Food Chem. 2011, 59, 12830–12835. [Google Scholar] [CrossRef]
- Charpentier, T.; Boisard, S.; Le Ray, A.M.; Bréard, D.; Chabrier, A.; Esselin, H.; Guilet, D.; Ripoll, C.; Richomme, P. A Descriptive Chemical Composition of Concentrated Bud Macerates through an Optimized SPE-HPLC-UV-MS2 Method—Application to Alnus glutinosa, Ribes nigrum, Rosa canina, Rosmarinus officinalis and Tilia tomentosa. Plants 2022, 11, 144. [Google Scholar] [CrossRef]
- D’Urso, G.; Maldini, M.; Pintore, G.; d’Aquino, L.; Montoro, P.; Pizza, C. Characterisation of Fragaria vesca fruit from Italy following a metabolomics approach through integrated mass spectrometry Techniques. LWT-Food Sci. Technol. 2016, 74, 387–395. [Google Scholar] [CrossRef]












| Molecular Formula | Name of Compound | R. fruticosus | R. nigrum | F. vesca | V. myrtillus |
|---|---|---|---|---|---|
| C7H6O5 | Gallic acid | x | x | x | x |
| C7H6O4 | Protocatechuic acid | x | x | ||
| C16H18O9 | Neochlorgenic acid | x | |||
| C15H18O9 | Caffeoyl hexoside (I) | x | |||
| C15H18O9 | Caffeoyl hexoside (II) | x | |||
| C15H18O9 | Caffeoyl hexoside (III) | x | |||
| C16H18O9 | Chlorogenic acid | x | x | x | x |
| C16H18O9 | Cryptochlorogenic acid | x | x | ||
| C9H8O3 | p-coumaric acid | x | |||
| C16H18O8 | p-coumaroylquinic acid | x | |||
| C19H14O12 | Ellagic acid pentoside | x | x | ||
| C25H28O13 | p-Coumaroyl monotropein | x | |||
| C14H6O8 | Ellagic acid | x | x | ||
| C27H30O16 | Quercetin rhamnoside 3-O-glucoside | x | |||
| C27H30O16 | Rutin | x | x | x | |
| C21H20O12 | Quercetin-3-O-galactoside | x | |||
| C21H18O13 | Quercetin-3-O-glucuronide | x | x | x | |
| C27H27O16 | Quercetin hydroxymethyl glutaroyl hexoside | x | |||
| C21H18O12 | Luteolin 3-O-glucoronide | x | |||
| C27H29O15 | Kaempferol-3-O-rutinoside | x | x | ||
| C24H22O15 | Quercetin malonyl glucoside | x | |||
| C21H20O11 | Kaempferol glucoside | x | |||
| C21H17O12 | Kaempferol glucuronide | x | |||
| C21H18O12 | Kaempferol-3-Oglucoronide | x | x | ||
| C21H17O12 | Kaempferol hexuronide | x | x | ||
| C21H18O12 | Dimethylellagic acid pentoside | x | |||
| C21H18O11 | Apigenin-3-O-glucoronide | x | |||
| C24H21O14 | Quercetin malonyl rhamnoside | x |
| Analyzed Plant | Name of Compound | Content (µg/mL) | ||
|---|---|---|---|---|
| Extract | F10 (10 Days) | F20 (20 Days) | ||
| Rubus fruticosus L. | Gallic acid | 3.99 ± 0.12 | 7.68 ± 0.06 | 6.04 ± 0.07 |
| Neochlorgenic acid | 1.05 ± 0.02 | 2.34 ± 0.00 | 2.77 ± 0.02 | |
| Caffeoyl hexoside (I) | 3.60 ± 0.01 | 7.13 ± 0.02 | 9.65 ± 0.10 | |
| Caffeoyl hexoside (II) | 6.58 ± 0.03 | 5.52 ± 0.01 | 4.45 ± 0.01 | |
| Caffeoyl hexoside (III) | 3.13 ± 0.03 | 5.82 ± 0.04 | 5.17 ± 0.02 | |
| Chlorogenic acid | 3.18 ± 0.01 | 5.84 ± 0.03 | 5.23 ± 0.01 | |
| Ellagic acid pentoside | 8.24 ± 0.42 | 23.27 ± 1.12 | 27.49 ± 0.65 | |
| Ellagic acid | 19.57 ± 0.86 | 80.87 ± 2.23 | 87.88 ± 0.42 | |
| Rutoside | 0.03 ± 0.00 | 2.23 ± 0.15 | 3.34 ± 0.09 | |
| Quercetin glucuronide | 7.12 ± 0.11 | 14.36 ± 0.21 | 21.68 ± 0.68 | |
| Luteolin-3-O-glucoronide | 6.12 ± 0.21 | 7.32 ± 0.07 | 8.89 ± 0.10 | |
| Kaempferol-3-O-rutinoside | 2.53 ± 0.01 | 4.54 ± 0.01 | 6.74 ± 0.04 | |
| Kaempferol-3-O-glucoronide | 6.86 ± 0.14 | 6.39 ± 0.13 | 5.94 ± 0.04 | |
| Apigenin-3-O-glucoronide | - | 12.38 ± 0.15 | 24.30 ± 1.14 | |
| Quercetin glucoside | 0.22 ± 0.02 | 2.86 ± 0.14 | 6.22 ± 0.13 | |
| Ribes nigrum L. | Gallic acid | 1.68 ± 0.01 | 6.55 ± 0.02 | 5.22 ± 0.02 |
| Protocatechuic acid | 6.56 ± 0.13 | 6.59 ± 0.01 | 6.54 ± 0.13 | |
| Chlorogenic acid | 8.84 ± 0.06 | 22.23 ± 0.57 | 21.22 ± 0.34 | |
| Cryptochlorogenic acid | 10.19 ± 0.54 | 11.68 ± 0.48 | 10.82 ± 0.30 | |
| p-coumaric acid | 6.03 ± 0.05 | 7.59 ± 0.07 | 7.46 ± 0.25 | |
| Rutin | 0.92 ± 0.01 | 25.13 ± 0.30 | 26.38 ± 0.28 | |
| Quercetin 3-glucoside | 3.82 ± 0.03 | 38.15 ± 0.12 | 38.46 ± 0.94 | |
| Quercetin malonyl glucoside | 11.95 ± 0.12 | 24.93 ± 0.17 | 23.63 ± 0.03 | |
| Kaempferol glucoside | 1.43 ± 0.08 | 20.75 ± 0.61 | 21.85 ± 0.06 | |
| Quercetin malonyl rhamnoside | 5.21 ± 0.06 | 7.99 ± 0.06 | 7.11 ± 0.15 | |
| Fragaria vesca L. | Gallic acid | 1.38 ± 0.05 | 15.85 ± 0.06 | 15.51 ± 0.47 |
| Chlorogenic acid | - | 4.72 ± 0.01 | 6.23 ± 0.05 | |
| Elagic acid | 9.97 ± 0.90 | 32.65 ± 0.78 | 75.58 ± 0.53 | |
| Dimethylellagic acid pentoside | 10.73 ± 0.28 | 10.01 ± 0.42 | 9.21 ± 0.53 | |
| Elagic acid pentozyd | 3.28 ± 0.13 | 21.68 ± 0.38 | 77.72 ± 1.12 | |
| Rutoside | - | 2.76 ± 0.18 | 4.23 ± 0.09 | |
| Quercetin glucuronide | 63.30 ± 0.00 | 56.37 ± 0.24 | 72.07 ± 0.05 | |
| Quercetin hydroxymethylglutaroyl hexoside | 12.00 ± 0.06 | 83.28 ± 0.26 | 161.73 ± 0.57 | |
| Kaempferol coumaroyl hexoside | 46.59 ± 0.74 | 21.51 ± 0.30 | - | |
| Kaempferolglucuronide | 65.78 ± 1.59 | 111.60 ± 3.10 | 177.44 ± 1.23 | |
| Vaccinum myrtillus L. | Gallic acid | - | 5.03 ± 0.14 | 2.70 ± 0.15 |
| Protocatechuic acid | 4.23 ± 0.09 | 5.32 ± 0.12 | 3.06 ± 0.15 | |
| Chlorogenic acid | 923.25 ± 1.43 | 1362.91 ± 8.88 | 1015.54 ± 1.83 | |
| Cryptochlorogenic acid | 54.94 ± 1.45 | 75.80 ± 0.65 | 61.51 ± 1.69 | |
| p-coumaroylquinic acid II | 5.40 ± 0.03 | 8.09 ± 0.10 | 6.80 ± 0.24 | |
| p-Coumaroyl monotropein | 11.27 ± 0.55 | 17.42 ± 0.28 | 13.57 ± 0.38 | |
| p-Coumaroyl diacetylhexoside | 26.68 ± 0.47 | 41.53 ± 1.02 | 31.20 ± 0.53 | |
| p-Coumaroyl malonylhexoside I | 5.83 ± 0.26 | 8.70 ± 0.14 | 6.67 ± 0.22 | |
| p-Coumaroyl malonylhexoside II | 30.67 ± 0.23 | 49.19 ± 0.24 | 40.18 ± 0.51 | |
| Quercetin galactoside | 11.90 ± 0.08 | 27.12 ± 1.33 | 17.75 ± 0.32 | |
| Quercetin hexuronide | 106.22 ± 0.23 | 172.68 ± 2.86 | 116.64 ± 1.06 | |
| Kaempferol glucuronide | 15.42 ± 0.37 | 23.49 ± 0.34 | 18.44 ± 0.34 | |
| Extract Plant | Ferment 10 Days | Ferment 20 Days | |
|---|---|---|---|
| Type of Analyzed Plant | IC50 (µg/mL) | ||
| Rubus fruticosus L. | 324.5 ± 0.25 | 96.2 ± 0.18 | 91.4 ± 0.17 |
| Ribes nigrum L. | 1215.7 ± 0.36 | 278.3 ± 0.17 | 351.8 ± 0.37 |
| Fragaria vesca L. | 1172.5 ±0.13 | 121.3 ± 0.02 | 114.7 ± 0.16 |
| Vaccinum myrtillus L. | 494.7 ± 0.31 | 118.3 ± 0.18 | 105.2 ± 0.08 |
| Extract Plant | Ferment 10 Days | Ferment 20 Days | |
|---|---|---|---|
| Type of Analyzed Plant | IC50 (µg/mL) | ||
| Rubus fruticosus L. | 92.3 ± 0.04 | 89.4 ± 0.08 | 90.4 ± 0.12 |
| Ribes nigrum L. | 97.9 ± 0.06 | 94.3 ± 0.15 | 95.6 ± 0.21 |
| Fragaria vesca L. | 97.3 ± 0.08 | 94.3 ± 0.14 | 93.7 ± 0.19 |
| Vaccinum myrtillus L. | 92.7 ± 0.21 | 93.5 ± 0.10 | 93.1 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Zagórska-Dziok, M.; Bujak, T.; Wójciak, M.; Sowa, I. Evaluation of Cosmetic and Dermatological Properties of Kombucha-Fermented Berry Leaf Extracts Considered to Be By-Products. Molecules 2022, 27, 2345. https://doi.org/10.3390/molecules27072345
Ziemlewska A, Nizioł-Łukaszewska Z, Zagórska-Dziok M, Bujak T, Wójciak M, Sowa I. Evaluation of Cosmetic and Dermatological Properties of Kombucha-Fermented Berry Leaf Extracts Considered to Be By-Products. Molecules. 2022; 27(7):2345. https://doi.org/10.3390/molecules27072345
Chicago/Turabian StyleZiemlewska, Aleksandra, Zofia Nizioł-Łukaszewska, Martyna Zagórska-Dziok, Tomasz Bujak, Magdalena Wójciak, and Ireneusz Sowa. 2022. "Evaluation of Cosmetic and Dermatological Properties of Kombucha-Fermented Berry Leaf Extracts Considered to Be By-Products" Molecules 27, no. 7: 2345. https://doi.org/10.3390/molecules27072345
APA StyleZiemlewska, A., Nizioł-Łukaszewska, Z., Zagórska-Dziok, M., Bujak, T., Wójciak, M., & Sowa, I. (2022). Evaluation of Cosmetic and Dermatological Properties of Kombucha-Fermented Berry Leaf Extracts Considered to Be By-Products. Molecules, 27(7), 2345. https://doi.org/10.3390/molecules27072345