High Yield Synthesis of Curcumin and Symmetric Curcuminoids: A “Click” and “Unclick” Chemistry Approach
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Synthon Preparation
4.2. Condensation of Aldehydes with the Synthon
4.3. General Methodology
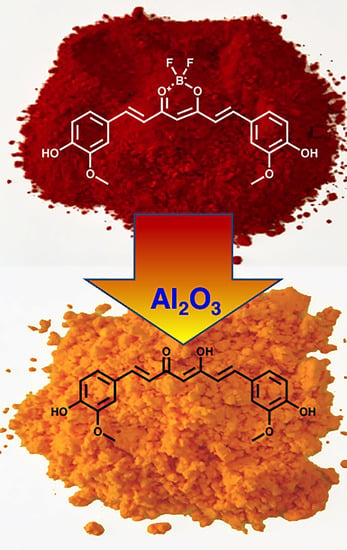
4.4. Reaction Conditions for “Unclick” Removal of the BF2 Group
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Meza-Morales, W.; Machado-Rodriguez, J.C.; Alvarez-Ricardo, Y.; Obregón-Mendoza, M.A.; Nieto-Camacho, A.; Toscano, R.A.; Soriano-García, M.; Cassani, J.; Enríquez, R.G. A new family of homoleptic copper complexes of curcuminoids: Synthesis, characterization and biological properties. Molecules 2019, 24, 910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obregón-Mendoza, M.A.; Arias-Olguín, I.I.; Estévez-Carmona, M.M.; Meza-Morales, W.; Alvarez-Ricardo, Y.; Toscano, R.A.; Arenas-Huertero, F.; Cassani, J.; Enríquez, R.G. Non-Cytotoxic Dibenzyl and Difluoroborate Curcuminoid Fluorophores Allow Visualization of Nucleus or Cytoplasm in Bioimaging. Molecules 2020, 25, 3205. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Gupta, P.; Singh, R.P.; Jafri, A.; Arshad, M.; Banerjee, M. Synthesis, spectroscopic characterization, theoretical study and anti-hepatic cancer activity study of 4-(1E,3Z,6E)-3-hydroxy-7-(4-hydroxy-3-methoxyphenyl)-5-oxohepta-1,3,6-trien-1-yl)-2-methoxyphenyl 4-nitrobenzoate, a novel curcumin congener. J. Mol. Struct. 2017, 1141, 678–686. [Google Scholar] [CrossRef]
- Mapoung, S.; Mapoung, S.; Suzuki, S.; Fuji, S.; Naiki-Ito, A.; Kato, H.; Yodkeeree, S.; Yodkeeree, S.; Sakorn, N.; Sakorn, N.; et al. Dehydrozingerone, a Curcumin Analog, as a Potential Anti-Prostate Cancer Inhibitor In Vitro and In Vivo. Molecules 2020, 25, 2737. [Google Scholar] [CrossRef]
- Chignell, C.F.; Bilski, P.; Reszka, K.J.; Motten, A.G.; Sik, R.H.; Dahl, T.A. Spectral and Photochemical Properties of Curcumin. Photochem. Photobiol. 1994, 59, 295–302. [Google Scholar] [CrossRef]
- Sumanont, Y.; Murakami, Y.; Tohda, M.; Vajragupta, O.; Watanabe, H.; Matsumoto, K. Effects of manganese complexes of curcumin and diacetylcurcumin on kainic acid-induced neurotoxic responses in the rat hippocampus. Biol. Pharm. Bull. 2007, 30, 1732–1739. [Google Scholar] [CrossRef] [Green Version]
- Parameswari, A.R.; Devipriya, B.; Jenniefer, S.J.; Muthiah, P.T.; Kumaradhas, P. Low temperature crystal structure of 5-hydroxy-1,7-bis-(4-hydroxy-3-Methoxy-phenyl)-hepta-1,6-dien-3-one. J. Chem. Crystallogr. 2012, 42, 227–231. [Google Scholar] [CrossRef]
- Price, L.C.; Buescher, R.W. Kinetics of alkaline degradation of the food pigments curcumin and curcuminoids. J. Food Sci. 1997, 62, 267–269. [Google Scholar] [CrossRef]
- Shehzad, A.; Lee, Y.S. Curcumin: Multiple molecular targets mediate multiple pharmacological actions—A review. Drugs Future 2010, 35, 113–119. [Google Scholar] [CrossRef]
- Feng, L.; Li, Y.; Song, Z.F.; Li, H.J.; Huai, Q.Y. Synthesis and biological evaluation of curcuminoid derivatives. Chem. Pharm. Bull. 2015, 63, 873–881. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Singh, V.; Shankar, R.; Kumar, K.; Rawal, R. Synthetic and medicinal prospective of structurally modified curcumins. Curr. Top. Med. Chem. 2016, 16, 1–14. [Google Scholar] [CrossRef]
- Ramirez-Ahumada, M.d.C.; Timmermann, B.N.; Gang, D.R. Biosynthesis of curcuminoids and gingerols in turmeric (Curcuma longa) and ginger (Zingiber officinale): Identification of curcuminoid synthase and hydroxycinnamoyl-CoA thioesterases. Phytochemistry 2006, 67, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Zamrus, S.N.H.; Akhtar, M.N.; Yeap, S.K.; Quah, C.K.; Loh, W.S.; Alitheen, N.B.; Zareen, S.; Tajuddin, S.N.; Hussin, Y.; Shah, S.A.A. Design, synthesis and cytotoxic effects of curcuminoids on HeLa, K562, MCF-7 and MDA-MB-231 cancer cell lines. Chem. Cent. J. 2018, 12, 31. [Google Scholar] [CrossRef]
- Jacob, J.N. Comparative studies in relation to the structure and biochemical properties of the active compounds in the volatile and nonvolatile fractions of turmeric (C. longa) and ginger (Z. officinale). Stud. Nat. Prod. Chem. 2016, 48, 101–135. [Google Scholar]
- Lin, L.; Lee, K.H. Structure-activity relationships of curcumin and its analogs with different biological activities. Stud. Nat. Prod. Chem. 2006, 33, 785–812. [Google Scholar]
- Banuppriya, G.; Sribalan, R.; Padmini, V. Synthesis and characterization of curcumin-sulfonamide hybrids: Biological evaluation and molecular docking studies. J. Mol. Struct. 2018, 1155, 90–100. [Google Scholar] [CrossRef]
- Medigue, N.E.H.; Bouakouk-Chitti, Z.; Bechohra, L.L.; Kellou-Taïri, S. Theoretical study of the impact of metal complexation on the reactivity properties of Curcumin and its diacetylated derivative as antioxidant agents. J. Mol. Model. 2021, 27, 192. [Google Scholar] [CrossRef]
- Banerjee, S.; Chakravarty, A.R. Metal complexes of curcumin for cellular imaging, targeting, and photoinduced anticancer activity. Acc. Chem. Res. 2015, 48, 2075–2083. [Google Scholar] [CrossRef]
- Obregón-Mendoza, M.A.; Estévez-Carmona, M.M.; Hernández-Ortega, S.; Soriano-García, M.; Ramírez-Apan, M.T.; Orea, L.; Pilotzi, H.; Gnecco, D.; Cassani, J.; Enríquez, R.G. Retro-curcuminoids as mimics of dehydrozingerone and curcumin: Synthesis, NMR, X-ray, and cytotoxic activity. Molecules 2017, 22, 33. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011, 28, 1937–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Wu, W.; Yu, F.; Huang, H.; Liang, X.; Ye, J. Catalytic asymmetric Michael addition with curcumin derivative. Org. Biomol. Chem. 2011, 9, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Ayyagari, N.; Jose, D.; Mobin, S.M.; Namboothiri, I.N.N. Stereoselective construction of carbocycles and heterocycles via cascade reactions involving curcumins and nitroalkenes. Tetrahedron Lett. 2011, 52, 258–262. [Google Scholar] [CrossRef]
- Ayyagari, N.; Namboothiri, I.N.N. Diastereo- and enantioselective synthesis of densely functionalized cyclohexanones via double Michael addition of curcumins with nitroalkenes. Tetrahedron Asymmetry 2012, 23, 605–610. [Google Scholar] [CrossRef]
- Urošević, M.; Nikolić, L.; Gajić, I.; Nikolić, V.; Dinić, A.; Miljković, V. Curcumin: Biological activities and modern pharmaceutical forms. Antibiotics 2022, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Devassy, J.G.; Nwachukwu, I.D.; Jones, P.J.H. Curcumin and cancer: Barriers to obtaining a health claim. Nutr. Rev. 2015, 73, 155–165. [Google Scholar] [CrossRef]
- Jalili-Nik, M.; Soltani, A.; Moussavi, S.; Ghayour-Mobarhan, M.; Ferns, G.A.; Hassanian, S.M.; Avan, A. Current status and future prospective of Curcumin as a potential therapeutic agent in the treatment of colorectal cancer. J. Cell. Physiol. 2018, 233, 6337–6345. [Google Scholar] [CrossRef]
- Zoi, V.; Galani, V.; Lianos, G.D.; Voulgaris, S.; Kyritsis, A.P.; Alexiou, G.A. The role of curcumin in cancer treatment. Biomedicines 2021, 9, 1086. [Google Scholar] [CrossRef]
- Kong, W.Y.; Ngai, S.C.; Goh, B.H.; Lee, L.H.; Htar, T.T.; Chuah, L.H. Is curcumin the answer to future chemotherapy cocktail? Molecules 2021, 26, 4329. [Google Scholar] [CrossRef]
- Badreldin, H.; Marrif, H.; Noreldayem, S.A.; Bakheit, A.O.; Blunden, G. Some biological properties of curcumin: A review. Nat. Prod. Commun. 2006, 1, 509–521. [Google Scholar]
- Sanphui, P.; Bolla, G. Curcumin, a Biological Wonder Molecule: A Crystal Engineering Point of View. Cryst. Growth Des. 2018, 18, 5690–5711. [Google Scholar] [CrossRef]
- Bandyopadhyay, D. Farmer to pharmacist: Curcumin as an anti-invasive and antimetastatic agent for the treatment of cancer. Front. Chem. 2014, 2, 113. [Google Scholar] [CrossRef]
- Mishra, S.; Palanivelu, K. The effect of curcumin (turmeric) on Alzheimer’s disease: An overview. Ann. Indian Acad. Neurol. 2008, 11, 13–19. [Google Scholar] [CrossRef]
- Boarescu, P.M.; Boarescu, I.; Bocșan, I.C.; Gheban, D.; Bulboacă, A.E.; Nicula, C.; Pop, R.M.; Râjnoveanu, R.M.; Bolboacă, S.D. Antioxidant and anti-inflammatory effects of curcumin nanoparticles on drug-induced acute myocardial infarction in diabetic rats. Antioxidants 2019, 8, 504. [Google Scholar] [CrossRef] [Green Version]
- Hussain, H.; Ahmad, S.; Shah, S.W.A.; Ullah, A.; Rahman, S.U.; Ahmad, M.; Almehmadi, M.; Abdulaziz, O.; Allahyani, M.; Alsaiari, A.A.; et al. Synthetic mono-carbonyl curcumin analogues attenuate oxidative stress in mouse models. Biomedicines 2022, 10, 2597. [Google Scholar] [CrossRef]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef]
- Witika, B.A.; Makoni, P.A.; Matafwali, S.K.; Mweetwa, L.L.; Shandele, G.C.; Walker, R.B. Enhancement of biological and pharmacological properties of an encapsulated polyphenol: Curcumin. Molecules 2021, 26, 4244. [Google Scholar] [CrossRef]
- Hussain, H.; Ahmad, S.; Shah, S.W.A.; Ullah, A.; Almehmadi, M.; Abdulaziz, O.; Allahyani, M.; Alsaiari, A.A.; Halawi, M.; Alamer, E. Investigation of antistress and antidepressant activities of synthetic curcumin analogues: Behavioral and biomarker approach. Biomedicines 2022, 10, 2385. [Google Scholar] [CrossRef]
- Hussain, H.; Ahmad, S.; Shah, S.W.A.; Ullah, A.; Ali, N.; Almehmadi, M.; Ahmad, M.; Khalil, A.A.K.; Jamal, S.B.; Ahmad, H.; et al. Attenuation of scopolamine-induced amnesia via cholinergic modulation in mice by synthetic curcumin analogs. Molecules 2022, 27, 2468. [Google Scholar] [CrossRef]
- Ahmed, T.; Gilani, A.H. Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimer’s disease. Pharmacol. Biochem. Behav. 2009, 91, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Hotsumi, M.; Makabe, K.; Konno, H. Design, synthesis and evaluation of curcumin-based fluorescent probes to detect Aβ fibrils. Bioorganic Med. Chem. Lett. 2018, 28, 3520–3525. [Google Scholar] [CrossRef] [PubMed]
- Chainoglou, E.; Hadjipavlou-Litina, D. Curcumin in health and diseases: Alzheimer’s disease and curcumin analogues, derivatives, and hybrids. Int. J. Mol. Sci. 2020, 21, 1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives: A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phyther. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef]
- Basile, V.; Ferrari, E.; Lazzari, S.; Belluti, S.; Pignedoli, F.; Imbriano, C. Curcumin derivatives: Molecular basis of their anti-cancer activity. Biochem. Pharmacol. 2009, 78, 1305–1315. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, F.C.; Anil Kumar, N.V.; Thakur, G. Developments in the anticancer activity of structurally modified curcumin: An up-to-date review. Eur. J. Med. Chem. 2019, 177, 76–104. [Google Scholar] [CrossRef]
- Jha, N.N.; Ghosh, D.; Das, S.; Anoop, A.; Jacob, R.S.; Singh, P.K.; Ayyagari, N.; Namboothiri, I.N.N.; Maji, S.K. Effect of curcumin analogs on α-synuclein aggregation and cytotoxicity. Sci. Rep. 2016, 6, 28511. [Google Scholar] [CrossRef] [Green Version]
- Sohn, S.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P. Biomedical Applications and Bioavailability of Curcumin—An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef]
- Linder, B.; Köhler, L.H.F.; Reisbeck, L.; Menger, D.; Subramaniam, D.; Herold-Mende, C.; Anant, S.; Schobert, R.; Biersack, B.; Kögel, D. A new pentafluorothio-substituted curcuminoid with superior antitumor activity. Biomolecules 2021, 11, 947. [Google Scholar] [CrossRef] [PubMed]
- Wanninger, S.; Lorenz, V.; Subhan, A.; Edelmann, F.T. Metal complexes of curcumin—Synthetic strategies, structures and medicinal applications. Chem. Soc. Rev. 2015, 44, 4986–5002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scotter, M.J. Synthesis and chemical characterisation of curcuminoid colouring principles for their potential use as HPLC standards for the determination of curcumin colour in foods. LWT—Food Sci. Technol. 2009, 42, 1345–1351. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.A.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin-from molecule to biological function. Angew. Chem. Int. Ed. 2012, 51, 5308–5332. [Google Scholar] [CrossRef] [PubMed]
- Miłobȩdzka, J.V.; Kostanecki, S.; Lampe, V. Zur Kenntnis des Curcumins. Ber. Dtsch. Chem. Ges. 1910, 43, 2163–2170. [Google Scholar] [CrossRef] [Green Version]
- Lampe, V. Synthese von Curcumin. Ber. Dtsch. Chem. Ges. 1918, 51, 1347–1355. [Google Scholar] [CrossRef] [Green Version]
- Pabon, H.J.J. A synthesis of curcumin and related compounds. Recl. Trav. Chim. Pays-Bas 1964, 83, 379–386. [Google Scholar] [CrossRef]
- Roughley, P.J.; Whiting, D.A. Experiments in the biosynhtesis of curcumin. J. Chem. Soc. Perkin Trans. 1973, 2379–2388. [Google Scholar] [CrossRef]
- Payton, F.; Sandusky, P.; Alworth, W.L. NMR study of the solution structure of curcumin. J. Nat. Prod. 2007, 70, 143–146. [Google Scholar] [CrossRef]
- Cooksey, C.J. Turmeric: Old spice, new spice. Biotech. Histochem. 2017, 92, 309–314. [Google Scholar] [CrossRef]
- Anjomshoa, S.; Namazian, M.; Noorbala, M.R. The Effect of Solvent on Tautomerism, Acidity and Radical Stability of Curcumin and Its Derivatives Based on Thermodynamic Quantities. J. Solut. Chem. 2016, 45, 1021–1030. [Google Scholar] [CrossRef]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Hassaninasab, A.; Hashimoto, Y.; Tomita-Yokotani, K.; Kobayashi, M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of curcumin: From mechanism to biological implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Xu, R.X.; Liu, Z. A high-throughput quantification method of curcuminoids and curcumin metabolites in human plasma via high-performance liquid chromatography/tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 949–950, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Péret-Almeida, L.; Cherubino, A.P.F.; Alves, R.J.; Dufossé, L.; Glória, M.B.A. Separation and determination of the physico-chemical characteristics of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Res. Int. 2005, 38, 1039–1044. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, E.; Hoehle, S.I.; Walch, S.G.; Riess, A.; Sólyom, A.M.; Metzler, M. Curcuminoids form reactive glucuronides in vitro. J. Agric. Food Chem. 2007, 55, 538–544. [Google Scholar] [CrossRef]
- Di Meo, F.; Filosa, S.; Madonna, M.; Giello, G.; Di Pardo, A.; Maglione, V.; Baldi, A.; Crispi, S. Curcumin C3 complex®/Bioperine® has antineoplastic activity in mesothelioma: An in vitro and in vivo analysis. J. Exp. Clin. Cancer Res. 2019, 38, 360. [Google Scholar] [CrossRef]
- Wichitnithad, W.; Nimmannit, U.; Wacharasindhu, S.; Rojsitthisak, P. Synthesis, characterization and biological evaluation of succinate prodrugs of curcuminoids for colon cancer treatment. Molecules 2011, 16, 1888–1900. [Google Scholar] [CrossRef] [Green Version]
- Venkata Rao, E.; Sudheer, P. Revisiting curcumin chemistry part I: A new strategy for the synthesis of curcuminoids. Indian J. Pharm. Sci. 2011, 73, 262–270. [Google Scholar]
- Titekar, R.V.; Hernández, M.; Land, D.P.; Nitin, N. “Click chemistry” based conjugation of lipophilic curcumin to hydrophilic ε-polylysine for enhanced functionality. Food Res. Int. 2013, 54, 44–47. [Google Scholar] [CrossRef]
- Weiss, H.; Reichel, J.; Görls, H.; Schneider, K.R.A.; Micheel, M.; Pröhl, M.; Gottschaldt, M.; Dietzek, B.; Weigand, W. Curcuminoid-BF2 complexes: Synthesis, fluorescence and optimization of BF2 group cleavage. Beilstein J. Org. Chem. 2017, 13, 2264–2272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polishchuk, V.; Stanko, M.; Kulinich, A.; Shandura, M. D–π–A–π–D Dyes with a 1,3,2-Dioxaborine Cycle in the Polymethine Chain: Efficient Long-Wavelength Fluorophores. Eur. J. Org. Chem. 2018, 2018, 240–246. [Google Scholar] [CrossRef]
- Bellinger, S.; Hatamimoslehabadi, M.; Bag, S.; Mithila, F.; La, J.; Frenette, M.; Laoui, S.; Szalda, D.J.; Yelleswarapu, C.; Rochford, J. Photophysical and Photoacoustic Properties of Quadrupolar Borondifluoride Curcuminoid Dyes. Chem.—A Eur. J. 2018, 24, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, J.; Chojnacki, J.; Zhang, S. BF3·OEt2-promoted concise synthesis of difluoroboron-derivatized curcumins from aldehydes and 2,4-pentanedione. Tetrahedron Lett. 2013, 54, 2070–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gál, E.; Csaba Nagy, L. Photophysical Properties and Electronic Structure of Symmetrical Curcumin Analogues and Their BF2 Complexes, Including a Phenothiazine Substituted Derivative. Symmetry 2021, 13, 2299. [Google Scholar] [CrossRef]
- Insuasty, D.; Cabrera, L.; Ortiz, A.; Insuasty, B.; Quiroga, J.; Abonia, R. Synthesis, photophysical properties and theoretical studies of new bis-quinolin curcuminoid BF2-complexes and their decomplexed derivatives. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2020, 230, 118065. [Google Scholar] [CrossRef]
- Laali, K.K.; Zwarycz, A.T.; Bunge, S.D.; Borosky, G.L.; Nukaya, M.; Kennedy, G.D. Deuterated Curcuminoids: Synthesis, Structures, Computational/Docking and Comparative Cell Viability Assays against Colorectal Cancer. ChemMedChem 2019, 14, 1173–1184. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Jiang, J.Q. Technologies for boron removal. Ind. Eng. Chem. Res. 2008, 47, 16–24. [Google Scholar] [CrossRef]
- Goldberg, S.; Glaubig, R.A. Boron Adsorption on Aluminum and Iron Oxide Minerals. Soil Sci. Soc. Am. J. 1985, 49, 1374–1379. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Hashimoto, A.; Igarashi, T.; Yoneda, T. Leaching of boron, arsenic and selenium from sedimentary rocks: II. pH dependence, speciation and mechanisms of release. Sci. Total Environ. 2014, 473–474, 244–253. [Google Scholar] [CrossRef] [PubMed]
- McPhail, M.; Page, L.A.; Bingham, F.T. Adsorption Interactions of Monosilicic and Boric Acid on Hydrous Oxides of Iron and Aluminum. Soil Sci. Soc. Am. J. 1972, 36, 510–514. [Google Scholar] [CrossRef]
- Toner, C.V.; Sparks, D.L. Chemical Relaxation and Double Layer Model Analysis of Boron Adsorption on Alumina. Soil Sci. Soc. Am. J. 1995, 59, 395–404. [Google Scholar] [CrossRef]
- Ranjbar, F.; Jalali, M. Surface complexation model of boron adsorption by calcareous soils. Int. J. Environ. Sci. Technol. 2014, 11, 1317–1326. [Google Scholar] [CrossRef] [Green Version]
- Sims, J.R.; Bingham, F.T. Retention of Boron by Layer Silicates, Sesquioxides, and Soil Materials: III. Iron- and Aluminum-Coated Layer Silicates and Soil Materials. Soil Sci. Soc. Am. J. 1968, 32, 369–373. [Google Scholar] [CrossRef]
- Martichonok, V.V.; Chiang, P.K.; Dornbush, P.J.; Land, K.M. On regioselectivity of aldol condensation of aromatic aldehydes with borate complex of acetylacetone. Synth. Commun. 2014, 44, 1245–1250. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem.—Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth Heinemann: Oxford, UK, 1997. [Google Scholar]
- MNova Software. Available online: https://mestrelab.com/download/mnova/ (accessed on 1 December 2022).
- Jayaprakasha, G.K.; Jagan Mohan Rao, L.; Sakariah, K.K. Improved HPLC method for the determination of curcumin, desmethoxycurcumin, and bisdesmethoxycurcumin. J. Agric. Food Chem. 2002, 50, 3668–3672. [Google Scholar] [CrossRef]







| Aldehyde | Curcuminoid-BF2 | Yield A |
|---|---|---|
| Vanillin | 1 | 90% |
| 4-hidroxybenzaldehyde | 2 | 85% |
| Furfural | 3 | 80% |
| 2-Thiophene carboxaldehyde | 4 | 95% |
| Curcuminoid-BF2 | Solvent | Metal Oxide | Time in Reflux (h) | Curcuminoid | Yield A |
|---|---|---|---|---|---|
| 1 | EtOH | BSilica | 72 | 5 (Curcumin) | 52% |
| 1 | EtOH | CMolecular sieves 4Å | 24 | 5 | 74% |
| 1 | EtOH | DAlumina | 24 | 5 | 60% |
| 1 | MeOH | Silica | 24 | 5 | 70% |
| 1 | MeOH | Molecular sieves 4Å | 24 | 5 | <80% |
| 1 | MeOH | Alumina | 24 | 5 | 85% |
| 2 | MeOH | Silica | 24 | 6(bis-demethoxycurcumin) | <50% |
| 2 | MeOH | Molecular sieves 4Å | 24 | 6 | 65% |
| 2 | MeOH | Alumina | 24 | 6 | 78% |
| 3 | MeOH | Silica | 72 | 7 | <30% |
| 3 | MeOH | Molecular sieves 4Å | 24 | 7 | 88% |
| 3 | MeOH | Alumina | 24 | 7 | 81% |
| 4 | MeOH | Silica | 72 | 8 | 60% |
| 4 | MeOH | Molecular sieves 4Å | 24 | 8 | 86% |
| 4 | MeOH | Alumina | 24 | 8 | 92% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obregón-Mendoza, M.A.; Meza-Morales, W.; Alvarez-Ricardo, Y.; Estévez-Carmona, M.M.; Enríquez, R.G. High Yield Synthesis of Curcumin and Symmetric Curcuminoids: A “Click” and “Unclick” Chemistry Approach. Molecules 2023, 28, 289. https://doi.org/10.3390/molecules28010289
Obregón-Mendoza MA, Meza-Morales W, Alvarez-Ricardo Y, Estévez-Carmona MM, Enríquez RG. High Yield Synthesis of Curcumin and Symmetric Curcuminoids: A “Click” and “Unclick” Chemistry Approach. Molecules. 2023; 28(1):289. https://doi.org/10.3390/molecules28010289
Chicago/Turabian StyleObregón-Mendoza, Marco A., William Meza-Morales, Yair Alvarez-Ricardo, M. Mirian Estévez-Carmona, and Raúl G. Enríquez. 2023. "High Yield Synthesis of Curcumin and Symmetric Curcuminoids: A “Click” and “Unclick” Chemistry Approach" Molecules 28, no. 1: 289. https://doi.org/10.3390/molecules28010289
APA StyleObregón-Mendoza, M. A., Meza-Morales, W., Alvarez-Ricardo, Y., Estévez-Carmona, M. M., & Enríquez, R. G. (2023). High Yield Synthesis of Curcumin and Symmetric Curcuminoids: A “Click” and “Unclick” Chemistry Approach. Molecules, 28(1), 289. https://doi.org/10.3390/molecules28010289