Iodine Biofortification of Dandelion Plants (Taraxacum officinale F.H. Wiggers Coll.) with the Use of Inorganic and Organic Iodine Compounds
Abstract
:1. Introduction
2. Results
2.1. Growth of Dandelion Plants
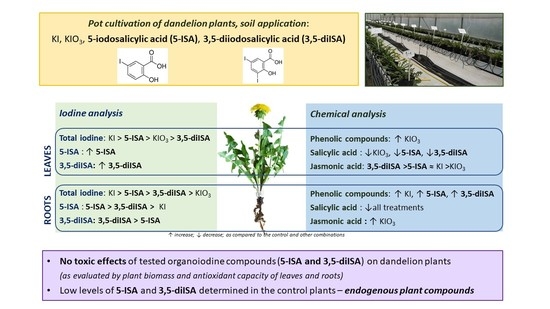
2.2. Iodine Content in Dandelion Roots and Leaves
2.3. The Content of Phenolic Compounds and Antioxidant Capacity of Dandelion Plants
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals Used
4.2. Experimental Design and Plant Cultivation
4.3. Sample Preparation
4.4. Total Iodine Analysis
4.5. Analysis of Salicylic, Iodosalicylic and Jasmonic Acids
4.6. Analysis of Total Phenolic Compounds
4.7. Analysis of Antioxidant Capacity
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zimmermann, M.B.; Jooste, P.L.; Pandav, C.S. Iodine-deficiency disorders. Lancet 2008, 372, 1251–1262. [Google Scholar] [CrossRef]
- Fuge, R.; Johnson, C.C. Iodine and human health, the role of environmental geochemistry and diet, a review. Appl. Geochem. 2015, 63, 282–302. [Google Scholar] [CrossRef]
- Fuge, R. Soils and Iodine Deficiency. In Essentials of Medical Geology: Revised Edition; Springer: Berlin/Heidelberg, Germany, 2013; pp. 417–432. [Google Scholar] [CrossRef] [Green Version]
- Shetaya, W.H.; Young, S.D.; Watts, M.J.; Ander, E.L.; Bailey, E.H. Iodine dynamics in soils. Geochim. Cosmochim. Acta 2012, 77, 457–473. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, S.; Kimble, G.; Schmett, G.; Emerson, D.; Turner, M.; Rudin, M. Abiotic reaction of iodate with sphagnum peat and other natural organic matter. J. Radioanal. Nucl. Chem. 2008, 277, 185–191. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Nakano, M.; Takamatsu, R.; Tanida, H. Inorganic iodine incorporation into soil organic matter: Evidence from iodine K-edge X-ray absorption near-edge structure. J. Environ. Radioact. 2010, 101, 451–457. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Kato, S.; Wachi, T.; Yoshihira, K.; Nakagawa, T.; Ishikawa, A.; Takagi, D.; Tezuka, A.; Yoshida, H.; Yoshida, S.; Sekimoto, H.; et al. Rice (Oryza sativa L.) roots have iodate reduction activity in response to iodine. Front. Plant Sci. 2013, 4, 227. [Google Scholar] [CrossRef] [Green Version]
- Barber, M.J.; Notton, B.A. Spinach nitrate reductase: Effects of ionic strength and pH on the full and partial enzyme activities. Plant Physiol. 1990, 93, 537–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzali, S.; Kiferle, C.; Perata, P. Iodine biofortification of crops: Agronomic biofortification. metabolic engineering and iodine bioavailability. Curr. Opin. Biotechnol. 2017, 44, 16–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiferle, C.; Martinelli, M.; Salzano, A.M.; Gonzali, S.; Beltrami, S.; Salvadori, P.A.; Hora, K.; Holwerda, H.T.; Scaloni, A.; Perata, P. Evidences for a nutritional role of iodine in plants. Front. Plant Sci. 2021, 12, 616868. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Singh, K.; Iqbal, N.; Niska, N.; Rani, A.; Kumar, M.; Khatri, N.; Siddiqui, M.H.; Kim, S.T.; Attila, F.; et al. Iodine: An emerging biostimulant of growth and stress responses in plants. Plant Soil 2022, 486, 119–133. [Google Scholar] [CrossRef]
- Medrano-Macías, J.; Leija-Martínez, P.; González-Morales, S.; Juárez-Maldonado, A.; Benavides-Mendoza, A. Use of iodine to biofortify and promote growth and stress tolerance in crops. Front. Plant Sci. 2016, 7, 1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, P.H.; Zhao, F.J.; Dobermann, A. What is a plant nutrient? Changing definitions to advance science and innovation in plant nutrition. Plant Soil 2022, 476, 11–23. [Google Scholar] [CrossRef]
- EL Sabagh, A.; Islam, M.S.; Hossain, A.; Iqbal, M.A.; Mubeen, M.; Waleed, M.; Reginato, M.; Battaglia, M.; Ahmed, S.; Rehman, A.; et al. Phytohormones as growth regulators during abiotic stress tolerance in plants. Front. Agron. 2022, 4, 765068. [Google Scholar] [CrossRef]
- Buturi, C.V.; Mauro, R.P.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Mineral biofortification of vegetables as a tool to improve human diet. Foods 2021, 10, 223. [Google Scholar] [CrossRef]
- Halka, M.; Klimek-Chodacka, M.; Smoleń, S.; Baranski, R.; Ledwożyw-Smoleń, I.; Sady, W. Organic iodine supply affects tomato plants differently than inorganic iodine. Physiol. Plant. 2018, 164, 290–306. [Google Scholar] [CrossRef]
- Smoleń, S.; Czernicka, M.; Kowalska, I.; Kȩska, K.; Halka, M.; Grzebelus, D.; Grzanka, M.; Skoczylas, Ł.; Pitala, J.; Koronowicz, A.; et al. New aspects of uptake and metabolism of non-organic and organic iodine compounds—The role of vanadium and plant-derived thyroid hormone analogs in lettuce. Front. Plant Sci. 2021, 12, 653168. [Google Scholar] [CrossRef]
- Grzanka, M.; Smoleń, S.; Skoczylas, Ł.; Grzanka, D. Synthesis of Organic Iodine Compounds in Sweetcorn under the Influence of Exogenous Foliar Application of Iodine and Vanadium. Molecules 2022, 27, 1822. [Google Scholar] [CrossRef]
- Sularz, O.; Koronowicz, A.; Smoleń, S.; Boycott, C.; Stefanska, B. Iodine-biofortified lettuce can promote mitochondrial dependent pathway of apoptosis in human gastrointestinal cancer cells. Int. J. Mol. Sci. 2023, 24, 9869. [Google Scholar] [CrossRef]
- Puccinelli, M.; Landini, M.; Maggini, R.; Pardossi, A.; Incrocci, L. Iodine biofortification of sweet basil and lettuce grown in two hydroponic systems. Sci. Hortic. 2021, 276, 109783. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Pintimalli, L.; Rosellini, I.; Pezzarossa, B. Biofortification of Lettuce and Basil Seedlings to Produce Selenium Enriched Leafy Vegetables. Horticulturae 2022, 8, 801. [Google Scholar] [CrossRef]
- Newman, R.G.; Moon, Y.; Sams, C.E.; Tou, J.C.; Waterland, N.L. Biofortification of sodium selenate improves dietary mineral contents and antioxidant capacity of culinary herb microgreens. Front. Plant Sci. 2021, 12, 716437. [Google Scholar] [CrossRef] [PubMed]
- D’Imperio, M.; Renna, M.; Cardinali, A.; Buttaro, D.; Serio, F.; Santamaria, P. Calcium biofortification and bioaccessibility in soilless “baby leaf” vegetable production. Food Chem. 2016, 213, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, M.; Mehrjerdi, M.Z.; Karimi, S.; Tavallali, V. Using gypsum and selenium foliar application for mineral biofortification and improving the bioactive compounds of garlic ecotypes. Ind. Crops Prod. 2020, 154, 112742. [Google Scholar] [CrossRef]
- Shafiq, M.; Qadir, A.; Ahmad, S.R. Biofortification: A sustainable agronomic strategy to increase selenium content and antioxidant activity in garlic. Appl. Ecol. Environ. Res. 2019, 17, 1685–1704. [Google Scholar] [CrossRef]
- Pérez, M.B.; Lipinski, V.M.; Filippini, M.F.; Chacón-Madrid, K.; Arruda, M.A.Z.; Wuilloud, R.G. Selenium biofortification on garlic growth and other nutrients accumulation. Hortic. Bras. 2019, 37, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Kiferle, C.; Ascrizzi, R.; Martinelli, M.; Gonzali, S.; Mariotti, L.; Pistelli, L.; Flamini, G.; Perata, P. Effect of Iodine treatments on Ocimum basilicum L.: Biofortification. phenolics production and essential oil composition. PLoS ONE 2019, 14, e0226559. [Google Scholar] [CrossRef]
- Incrocci, L.; Carmassi, G.; Maggini, R.; Poli, C.; Saidov, D.; Tamburini, C.; Kiferle, C.; Perata, P.; Pardossi, A. Iodine accumulation and tolerance in sweet basil (Ocimum basilicum L.) with green or purple leaves grown in floating system technique. Front. Plant Sci. 2019, 10, 1494. [Google Scholar] [CrossRef]
- Golubkina, N.; Moldovan, A.; Fedotov, M.; Kekina, H.; Kharchenko, V.; Folmanis, G.; Alpatov, A.; Caruso, G. Iodine and Selenium Biofortification of Chervil Plants Treated with Silicon Nanoparticles. Plants 2021, 10, 2528. [Google Scholar] [CrossRef]
- Hu, C. Taraxacum: Phytochemistry and health benefits. Chin. Herb. Med. 2018, 10, 353–361. [Google Scholar] [CrossRef]
- Küpper, F.C.; Feiters, M.C.; Olofsson, B.; Kaiho, T.; Yanagida, S.; Zimmermann, M.B.; Carpenter, L.J.; Luther, G.W., III; Lu, Z.; Jonsson, M.; et al. Commemorating two centuries of iodine research: An interdisciplinary overview of current research. Angew. Chem. Int. Ed. 2011, 50, 11598–11620. [Google Scholar] [CrossRef] [PubMed]
- Blasco, B.; Ríos, J.J.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.M.; Leyva, R.; Romero, L.; Ruiz, J.M. Study of the interactions between iodine and mineral nutrients in lettuce plants. J. Plant Nutr. 2012, 35, 1958–1969. [Google Scholar] [CrossRef]
- Grzanka, M.; Smoleń, S.; Kováčik, P. Effect of vanadium on the uptake and distribution of organic and inorganic forms of iodine in sweetcorn plants during early-stage development. Agronomy 2020, 10, 1666. [Google Scholar] [CrossRef]
- Smoleń, S.; Ledwożyw-Smoleń, I.; Halka, M.; Sady, W.; Kováčik, P. The absorption of iodine from 5-iodosalicylic acid by hydroponically grown lettuce. Sci. Hortic. 2017, 225, 716–725. [Google Scholar] [CrossRef]
- Sularz, O.; Smoleń, S.; Koronowicz, A.; Kowalska, I.; Leszczyńska, T. Chemical Composition of Lettuce (Lactuca sativa L.) Biofortified with Iodine by KIO3, 5-Iodo-, and 3,5-Diiodosalicylic Acid in a Hydroponic Cultivation. Agronomy 2020, 10, 1022. [Google Scholar] [CrossRef]
- Dai, J.L.; Zhu, Y.G.; Zhang, M.; Huang, Y.Z. Selecting iodine-enriched vegetables and the residual effect of iodate application to soil. Biol. Trace Elem. Res. 2004, 101, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Caffagni, A.; Arru, L.; Meriggi, P.; Milc, J.; Perata, P.; Pecchioni, N. Iodine fortification plant screening process and accumulation in tomato fruits and potato tubers. Commun. Soil Sci. Plant Anal. 2011, 42, 706–718. [Google Scholar] [CrossRef]
- Smoleń, S.; Kowalska, I.; Skoczylas, Ł.; Tabaszewska, M.; Pitala, J.; Mrożek, J.; Kováčik, P. Effectiveness of enriching lettuce with iodine using 5-iodosalicylic and 3, 5-diiodosalicylic acids and the chemical composition of plants depending on the type of soil in a pot experiment. Food Chem. 2022, 382, 132347. [Google Scholar] [CrossRef]
- Halka, M.; Smoleń, S.; Ledwożyw-Smoleń, I.; Sady., W. Comparison of effects of potassium iodide and iodosalicylates on the antioxidant potential and iodine accumulation in young tomato plants. J. Plant Growth Regul. 2020, 39, 282–295. [Google Scholar] [CrossRef] [Green Version]
- Küpper, F.C.; Carpenter, L.J.; McFiggans, G.B.; Palmer, C.J.; Waite, T.J.; Boneberg, E.M.; Woitsch, S.; Weiller, M.; Abela, R.; Grolimund, D.; et al. Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry. Proc. Natl. Acad. Sci. USA 2008, 105, 6954–6958. [Google Scholar] [CrossRef]
- Leyva, R.; Sánchez-Rodríguez, E.; Ríos, J.J.; Rubio-Wilhelmi, M.M.; Romero, L.; Ruiz, J.M.; Blasco, B. Beneficial effects of exogenous iodine in lettuce plants subjected to salinity stress. Plant Sci. 2011, 181, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Bajpai, M.S.; Majumdar, R.S.; Mishra, P.K. Response of iodine on antioxidant levels of Glycine max L. grown under Cd2+ stress. Adv. Biol. Res. 2015, 9, 40–48. [Google Scholar]
- Blasco, B.; Rios, J.J.; Cervilla, L.M.; Sánchez-Rodrigez, E.; Ruiz, J.M.; Romero, L. Iodine biofortification and antioxidant capacity of lettuce: Potential benefits for cultivation and human health. Ann. Appl. Biol. 2008, 152, 289–299. [Google Scholar] [CrossRef]
- Blasco, B.; Ríos, J.J.; Leyva, R.; Cervilla, L.M.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.M.; Rosales, M.A.; Romero, L. Does iodine biofortification affect oxidative metabolism in lettuce plants? Biol. Trace Elem. Res. 2011, 142, 831–842. [Google Scholar] [CrossRef]
- Dávila Rangel, I.E.; Trejo Téllez, L.I.; Ortega Ortiz, H.; Juárez Maldonado, A.; González Morales, S.; Companioni González, B.; Cabrera de la Fuente, M.C.; Benavides Mendoza, A. Comparison of iodide, iodate, and iodine-chitosan complexes for the biofortification of lettuce. Appl. Sci. 2020, 10, 2378. [Google Scholar] [CrossRef] [Green Version]
- Krzepiłko, A.; Święciło, A.; Zych-Wężyk, I. The antioxidant properties and biological quality of radish seedlings biofortified with iodine. Agronomy 2021, 11, 2011. [Google Scholar] [CrossRef]
- Halka, M.; Smoleń, S.; Ledwożyw-Smoleń, I.; Sady, W. Iodosalicylates and iodobenzoates supplied to tomato plants affect the antioxidative and sugar metabolism differently than potassium iodide. Folia Hortic. 2019, 31, 385–400. [Google Scholar] [CrossRef] [Green Version]
- Sularz, O.; Koronowicz, A.; Smoleń, S.; Kowalska, I.; Skoczylas, Ł.; Liszka-Skoczylas, M.; Tabaszewska, M.; Pitala, J. Anti-and pro-oxidant potential of lettuce (Lactuca sativa L.) biofortified with iodine by KIO3, 5-iodo-and 3,5-diiodosalicylic acid in human gastrointestinal cancer cell lines. RSC Adv. 2021, 11, 27547–27560. [Google Scholar] [CrossRef]
- Ledwożyw-Smoleń, I.; Halka, M.; Smoleń, S.; Kruczek, M. Antioxidant potential of tomato (Solanum lycopersicum L.) seedlings as affected by the exogenous application of organoiodine compounds. Acta Sci. Pol. Hortorum Cultus 2020, 19, 3–15. [Google Scholar] [CrossRef]
- Halka, M.; Smoleń, S.; Czernicka, M.; Klimek-Chodacka, M.; Pitala, J.; Tutaj, K. Iodine biofortification through expression of HMT, SAMT and S3H genes in Solanum lycopersicum L. Plant Physiol. Biochem. 2019, 144, 35–48. [Google Scholar] [CrossRef]
- Ghasemi Pirbalouti, A.; Sajjadi, S.E.; Parang, K. A review (research and patents) on jasmonic acid and its derivatives. Arch. Pharm. Chem. Life Sci. 2014, 347, 229–239. [Google Scholar] [CrossRef]
- Ghorbel, M.; Brini, F.; Sharma, A.; Landi, M. Role of jasmonic acid in plants: The molecular point of view. Plant Cell Rep. 2021, 40, 1471–1494. [Google Scholar] [CrossRef] [PubMed]
- Swain, T.; Hillis, W.E. Phenolic constituents of Prunus domestica. I. Quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–71. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Apak, R.; Güclü, K.; Özyürek, M.; Esin Karademir, S. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2010, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
| Combination | Leaf Weight per Plant (g) | Root Weight per Plant (g) | Total Weight of a Plant (g) | % d.m. Leaves | % d.m. Roots |
|---|---|---|---|---|---|
| Control | 20.3 ± 0.87 a 1 | 37.9 ± 12.33 a | 58.2 ± 12.03 a | 14.3 ± 0.02 c | 21.6 ± 0.24 cd |
| KIO3 10 µM | 18.8 ± 0.95 a | 48.8 ± 3.47 a | 67.5 ± 4.11 a | 13.1 ± 0.03 a | 22.8 ± 0.27 d |
| KIO3 50 µM | 16.3 ± 1.03 a | 46.3 ± 2.95 a | 62.5 ± 3.86 a | 13.1 ± 0.06 a | 23.3 ± 0.23 d |
| KI 10 µM | 19.5 ± 0.65 a | 54.0 ± 9.06 a | 73.5 ± 9.18 a | 13.7 ± 0.04 b | 19.8 ± 0.44 bc |
| KI 50 µM | 19.3 ± 1.84 a | 70.8 ± 18.60 a | 90.0 ± 20.12 a | 15.1 ± 0.09 e | 15.9 ± 0.54 a |
| 5-ISA 10 µM | 19.0 ± 1.78 a | 70.3 ± 21.12 a | 89.3 ± 22.00 a | 14.3 ± 0.06 c | 22.0 ± 0.25 cd |
| 5-ISA 50 µM | 19.3 ± 1.55 a | 60.5 ± 12.81 a | 79.8 ± 13.60 a | 13.5 ± 0.04 b | 18.4 ± 0.75 b |
| 3,5-diISA 10 µM | 22.5 ± 1.85 a | 77.5 ± 18.46 a | 100.0 ± 19.79 a | 14.7 ± 0.06 d | 20.4 ± 0.81 bc |
| 3,5-diISA 50 µM | 20.0 ± 1.47 a | 65.5 ± 13.74 a | 85.5 ± 14.09 a | 14.3 ± 0.04 c | 19.1 ± 0.47 b |
| Combination | Leaves | Roots | ||||
|---|---|---|---|---|---|---|
| Iodine (mg I kg−1) | 5-ISA (ng g−1) | 3.5-diISA (ng g−1) | Iodine (mg I kg−1) | 5-ISA (ng g−1) | 3.5-diISA (ng g−1) | |
| Control | 0.3 ± 0.01 a 1 | 0.32 ± 0.03 a | 0.20 ± 0.67 a | 0.2 ± 0.01 a | 0.1. ± 0.03 a | 0.7 ± 0.20 a |
| KIO3 10 µM | 7.8 ± 0.08 b | 0.08 ± 0.02 a | 0.24 ± 0.09 a | 1.7 ± 0.02 b | 0.6 ± 0.14 abc | 1.2 ± 0.26 a |
| KIO3 50 µM | 49.6 ± 0.77 e | 0.12 ± 0.06 a | 0.56 ± 0.22 a | 9.1 ± 0.22 d | 0.3 ± 0.03 ab | 1.1 ± 0.10 a |
| KI 10 µM | 13.4 ± 0.47 c | 0.31 ± 0.05 a | 0.31 ± 0.08 a | 2.8 ± 0.04 c | 0.1 ± 0.02 a | 0.9 ± 0.15 a |
| KI 50 µM | 133.5 ± 1.89 g | 0.13 ± 0.05 a | 0.27 ± 0.08 a | 19.1 ± 0.15 f | 0.8 ± 0.17 bc | 0.3 ± 0.05 a |
| 5-ISA 10 µM | 9.2 ± 0.12 bc | 0.46 ± 0.14 a | 0.38 ± 0.16 a | 2.3 ± 0.01 bc | 0.9 ± 0.08 cd | 0.3 ± 0.05 a |
| 5-ISA 50 µM | 99.2 ± 1.74 f | 3.84 ± 0.21 b | 0.34 ± 0.12 a | 17.1 ± 0.44 e | 6.3 ± 0.18 e | 21.7 ± 0.54 b |
| 3,5-diISA 10 µM | 7.2 ± 0.12 b | 0.46 ± 0.12 a | 2.29 ± 0.23 b | 2.9 ± 0.02 c | 1.0 ± 0.05 cd | 22.2 ± 0.43 b |
| 3,5-diISA 50 µM | 38.5 ± 0.31 d | 0.40 ± 0.03 a | 8.88 ± 0.54 c | 9.1 ± 0.13 d | 1.4 ± 0.19 d | 95.8 ± 1.51 c |
| Combination | Phenolic Compounds (mg CAE g−1) | SA Content (ng g−1) | JA Content (ng g−1) |
|---|---|---|---|
| Control | 41.9 ± 0.48 bc 1 | 58.6 ± 1.56 c | 83.2 ± 9.35 a |
| KIO3 10 µM | 43.5 ± 0.54 c | 41.1 ± 1.41 ab | 122.6 ± 7.36 b |
| KIO3 50 µM | 50.2 ± 1.11 d | 47.3 ± 1.03 b | 120.4 ± 1.28 b |
| KI 10 µM | 37.2 ± 0.423 a | 43.6 ± 0.97 ab | 156.5 ± 4.78 cd |
| KI 50 µM | 39.5 ± 1.05 abc | 69.0 ± 1.44 d | 132.2 ± 2.54 bc |
| 5-ISA 10 µM | 43.7 ± 0.14 c | 41.5 ± 1.70 ab | 138.3 ± 6.48 bc |
| 5-ISA 50 µM | 36.5 ± 1.45 a | 38.4 ± 0.62 a | 164.6 ± 3.26 de |
| 3,5-diISA 10 µM | 38.8 ± 0.59 ab | 45.2 ± 1.89 b | 182.4 ± 4.25 e |
| 3,5-diISA 50 µM | 39.8 ± 1.29 abc | 42.2 ± 0.80 ab | 175.2 ± 2.92 de |
| Combination | Phenolic Compounds (mg CAE g−1) | SA Content (ng g−1) | JA Content (ng g−1) |
|---|---|---|---|
| Control | 8.11 ± 0.10 a 1 | 6.0 ± 0.14 d | 383.1 ± 3.63 f |
| KIO3 10 µM | 8.0 ± 0.17 a | 2.5 ± 0.12 a | 432.0 ± 1.42 g |
| KIO3 50 µM | 8.6 ± 0.19 ab | 4.3 ± 0.23 bc | 439.3 ± 3.44 g |
| KI 10 µM | 10.2 ± 0.13 d | 4.5 ± 0.26 c | 112.1 ± 3.55 a |
| KI 50 µM | 11.7 ± 0.12 e | 3.6 ± 0.11 b | 157.5 ± 1.64 b |
| 5-ISA 10 µM | 8.6 ± 0.06 ab | 5.1 ± 0.30 c | 258.7 ± 10.60 e |
| 5-ISA 50 µM | 9.0 ± 0.11 bc | 5.2 ± 0.22 cd | 190.9 ± 0.49 c |
| 3,5-diISA 10 µM | 8.14 ± 0.13 a | 4.6 ± 0.03 c | 218.4 ± 3.11 d |
| 3,5-diISA 50 µM | 9.7 ± 0.16 cd | 2.4 ± 0.15 a | 166.9 ± 2.57 b |
| Combination | Antioxidant Capacity (µmol TE g−1) | ||
|---|---|---|---|
| CUPRAC | FRAP | DPPH | |
| Control | 187.1 ± 2.66 a 1 | 150.6 ± 3.25 a | 67.8 ± 2.09 a |
| KIO3 10 µM | 201.4 ± 6.09 abc | 153.9 ± 2.15 ab | 71.1 ± 1.32 ab |
| KIO3 50 µM | 204.5 ± 4.09 abc | 168.4 ± 3.08 bc | 72.8 ± 1.88 b |
| KI 10 µM | 209.2 ± 2.89 bc | 173.7 ± 1.92 c | 69.7 ± 0.12 ab |
| KI 50 µM | 194.8 ± 2.74 ab | 164.6 ± 4.48 abc | 70.0 ± 0.90 ab |
| 5-ISA 10 µM | 201.2 ± 1.73 abc | 171.6 ± 5.10 c | 71.2 ± 1.00 ab |
| 5-ISA 50 µM | 197.0 ± 4.21 abc | 170.8 ± 1.21 c | 69.3 ± 0.66 ab |
| 3,5-diISA 10 µM | 214.6 ± 4.25 c | 178.8 ± 4.23 c | 67.2 ± 1.62 ab |
| 3,5-diISA 50 µM | 215.1 ± 4.66 c | 180.8 ± 3.40 c | 66.1 ± 1.14 a |
| Combination | Antioxidant Capacity (µmol TE g−1) | ||
|---|---|---|---|
| CUPRAC | FRAP | DPPH | |
| Control | 68.8 ±3.06 abc 1 | 24.4 ± 0.27 ab | 23.7 ± 0.69 ab |
| KIO3 10 µM | 66.9 ± 1.77 ab | 23.9 ± 0.44 a | 25.3 ± 0.82 abc |
| KIO3 50 µM | 76.4 ± 1.45 cd | 26.8 ± 0.29 cd | 26.6 ± 0.61 bc |
| KI 10 µM | 84.0 ± 0.93 d | 31.9 ± 0.57 e | 30.9 ± 0.27 d |
| KI 50 µM | 85.2 ± 2.11 d | 34.8 ± 0.40 f | 28.7 ± 0.65 cd |
| 5-ISA 10 µM | 71.1 ± 1.38 bc | 26.1 ± 0.46 bc | 24.9 ± 0.63 ab |
| 5-ISA 50 µM | 67.3 ± 1.78 ab | 25.8 ± 0.60 abc | 23.7 ± 0.79 ab |
| 3,5-diISA 10 µM | 61.8 ± 1.11 a | 24.1 ± 0.45 ab | 22.5 ± 0.37 a |
| 3,5-diISA 50 µM | 72.2 ± 2.54 bc | 28.8 ± 0.50 d | 27.2 ± 1.54 bcd |
| pH | EC | Eh | N-NH4+ | N-NO3− | Ca | K | Mg | Na | P | S | Cl− | I |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mS/cm | mV | mg dm−3 | mg·kg−1 | |||||||||
| 4.01 | 0.26 | 383.8 | 96.8 | 83.7 | 1913.3 | 196.2 | 72.6 | 20.9 | 12.3 | 1705.3 | 13.6 | 2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ledwożyw-Smoleń, I.; Pitala, J.; Smoleń, S.; Liszka-Skoczylas, M.; Kováčik, P. Iodine Biofortification of Dandelion Plants (Taraxacum officinale F.H. Wiggers Coll.) with the Use of Inorganic and Organic Iodine Compounds. Molecules 2023, 28, 5638. https://doi.org/10.3390/molecules28155638
Ledwożyw-Smoleń I, Pitala J, Smoleń S, Liszka-Skoczylas M, Kováčik P. Iodine Biofortification of Dandelion Plants (Taraxacum officinale F.H. Wiggers Coll.) with the Use of Inorganic and Organic Iodine Compounds. Molecules. 2023; 28(15):5638. https://doi.org/10.3390/molecules28155638
Chicago/Turabian StyleLedwożyw-Smoleń, Iwona, Joanna Pitala, Sylwester Smoleń, Marta Liszka-Skoczylas, and Peter Kováčik. 2023. "Iodine Biofortification of Dandelion Plants (Taraxacum officinale F.H. Wiggers Coll.) with the Use of Inorganic and Organic Iodine Compounds" Molecules 28, no. 15: 5638. https://doi.org/10.3390/molecules28155638
APA StyleLedwożyw-Smoleń, I., Pitala, J., Smoleń, S., Liszka-Skoczylas, M., & Kováčik, P. (2023). Iodine Biofortification of Dandelion Plants (Taraxacum officinale F.H. Wiggers Coll.) with the Use of Inorganic and Organic Iodine Compounds. Molecules, 28(15), 5638. https://doi.org/10.3390/molecules28155638