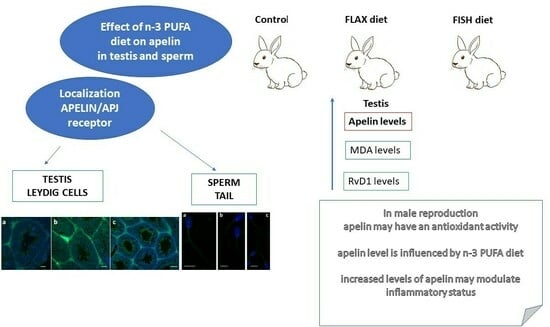
Can Dietary n-3 Polyunsaturated Fatty Acids Affect Apelin and Resolvin in Testis and Sperm of Male Rabbits?
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Testosterone Evaluation in Blood Serum
4.3. Semen Quality Assessment
4.4. Oxidative Status of Testis and Sperm
4.5. Fatty Acid Profiles of Diets, Testis and Sperm
4.6. Immunofluorescence in Testis and Sperm
4.7. Resolvin (Rv) D1 and Apelin Assays in Testis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Collodel, G.; Castellini, C.; Lee, J.C.; Signorini, C. Relevance of Fatty Acids to Sperm Maturation and Quality. Oxid. Med. Cell Longev. 2020, 2020, 7038124. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; Mattioli, S.; Signorini, C.; Cotozzolo, E.; Noto, D.; Moretti, E.; Brecchia, G.; Dal Bosco, A.; Belmonte, G.; Durand, T.; et al. Effect of Dietary n-3 Source on Rabbit Male Reproduction. Oxid. Med. Cell Longev. 2019, 2019, 3279670. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, S.; Dimauro, C.; Cesarani, A.; Dal Bosco, A.; Bartolini, D.; Galli, F.; Migni, A.; Sebastiani, B.; Signorini, C.; Oger, C.; et al. A Dynamic Model for Estimating the Interaction of ROS–PUFA–Antioxidants in Rabbit. Antioxidants 2022, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; Mattioli, S.; Moretti, E.; Cotozzolo, E.; Perini, F.; Dal Bosco, A.; Signorini, C.; Noto, D.; Belmonte, G.; Lasagna, E.; et al. Expression of genes and localization of enzymes involved in polyunsaturated fatty acid synthesis in rabbit testis and epididymis. Sci. Rep. 2022, 12, 2637. [Google Scholar] [CrossRef]
- Elfassy, Y.; McAvoy, C.; Fellahi, S.; Dupont, J.; Fève, B.; Levy, R.; Bastard, J.P. Seminal plasma adipokines: Involvement in human reproductive functions. Eur Cytokine Netw. 2017, 28, 141–150. [Google Scholar] [CrossRef]
- Singh, A.; Choubey, M.; Bora, P.; Krishna, A. Adiponectin and Chemerin: Contrary Adipokines in Regulating Reproduction and Metabolic Disorders. Reprod. Sci. 2018, 25, 1462–1473. [Google Scholar] [CrossRef]
- Shokrollahi, B.; Shang, J.H.; Saadati, N.; Ahmad, H.I.; Yang, C.Y. Reproductive roles of novel adipokines apelin, visfatin, and irisin in farm animals. Theriogenology 2021, 172, 178–186. [Google Scholar] [CrossRef]
- Higuchi, K.; Masaki, T.; Gotoh, K.; Chiba, S.; Katsuragi, I.; Tanaka, K.; Kakuma, T.; Yoshimatsu, H. Apelin, an APJ receptor ligand, regulates body adiposity and favors the messenger ribonucleic acid expression of uncoupling proteins in mice. Endocrinology 2007, 148, 2690–2697. [Google Scholar] [CrossRef]
- Soliman, M.; Arafah, M. Apelin protect against multiple organ injury following hemorrhagic shock and decrease the inflammatory response. Int. J. Appl. Basic Med. Res. 2015, 5, 195–199. [Google Scholar] [CrossRef]
- Than, A.; Zhang, X.; Leow, M.K.; Poh, C.L.; Chong, S.K.; Chen, P. Apelin attenuates oxidative stress in human adipocytes. J. Biol. Chem. 2014, 289, 3763–3774. [Google Scholar] [CrossRef]
- Kurowska, P.; Barbe, A.; Różycka, M.; Chmielińska, J.; Dupont, J.; Rak, A. Apelin in Reproductive Physiology and Pathology of Different Species: A Critical Review. Int. J. Endocrinol. 2018, 2018, 9170480. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A. Effect of Omega-3 or Omega-6 Dietary Supplementation on Testicular Steroidogenesis, Adipokine Network, Cytokines, and Oxidative Stress in Adult Male Rats. Oxid. Med. Cell Longev. 2021, 2021, 5570331. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Gurusubramanian, G.; Roy, V.K. Postnatal developmental expression of apelin receptor proteins and its role in juvenile mice testis. J. Steroid Biochem. Mol. Biol. 2022, 224, 106178. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; Bulló, M.; Salas-Salvadó, J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: A systematic review of observational studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Signorini, C.; Corsaro, R.; Noto, D.; Tripodi, A.S.; Menchiari, A.; Micheli, L.; Ponchia, R.; Collodel, G. Apelin is found in human sperm and testis and is raised in inflammatory pathological conditions. Cytokine 2023, 169, 156281. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J.; Levy, B.D. Lipid mediators in the resolution of inflammation. Cold Spring Harb. Perspect. Biol. 2014, 7, a016311. [Google Scholar] [CrossRef]
- Duvall, M.G.; Levy, B.D. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur. J. Pharmacol. 2016, 785, 144–155. [Google Scholar] [CrossRef]
- Signorini, C.; Moretti, E.; Noto, D.; Micheli, L.; Ponchia, R.; Collodel, G. Fatty Acid Oxidation and Pro-Resolving Lipid Mediators Are Related to Male Infertility. Antioxidants 2022, 11, 107. [Google Scholar] [CrossRef]
- Marchiani, S.; Vignozzi, L.; Filippi, S.; Gurrieri, B.; Comeglio, P.; Morelli, A.; Danza, G.; Bartolucci, G.; Maggi, M.; Baldi, E. Metabolic syndrome-associated sperm alterations in an experimental rabbit model: Relation with metabolic profile, testis and epididymis gene expression and effect of tamoxifen treatment. Mol Cell Endocrinol. 2015, 401, 12–24. [Google Scholar] [CrossRef]
- Marco-Jiménez, F.; Vicente, J.S. Overweight in young males reduce fertility in rabbitmodel. PLoS ONE 2017, 12, e0180679. [Google Scholar] [CrossRef]
- Fan, J.L.; Castellini, C.; Page, C.P.; Spina, D. The rabbit as a biomedical model. In The Genetics and Genomics of the Rabbit; CABI: Wallingford, UK, 2021; pp. 310–325. [Google Scholar]
- Estienne, A.; Bongrani, A.; Reverchon, M.; Ramé, C.; Ducluzeau, P.H.; Froment, P.; Dupont, J. Involvement of Novel Adipokines, Chemerin, Visfatin, Resistin and Apelin in Reproductive Functions in Normal and Pathological Conditions in Humans and Animal Models. Int. J. Mol. Sci. 2019, 20, 4431. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Zhang, K.; Wang, Z.; Ma, X.; Liu, H.; Zhao, J.; Lu, W.; Wang, J. Dietary flaxseed oil and vitamin E improve semen quality via propionic acid metabolism. Front. Endocrinol. 2023, 14, 1139725. [Google Scholar] [CrossRef] [PubMed]
- Yuzbashian, E.; Zarkesh, M.; Asghari, G.; Hedayati, M.; Safarian, M.; Mirmiran, P.; Khalaj, A. Is apelin gene expression and concentration affected by dietary intakes? A systematic review. Crit. Rev. Food Sci. Nutr. 2018, 58, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Cao, J.; Chen, L. Apelin/APJ system: A novel therapeutic target for oxidative stress-related inflammatory diseases (Review). Int. J. Mol. Med. 2016, 37, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, A.; Cao, J.; Chen, L. Apelin/APJ system: An emerging therapeutic target for respiratory diseases. Cell Mol. Life Sci. 2020, 77, 2919–2930. [Google Scholar] [CrossRef]
- Agarwal, A.; Rana, M.; Qiu, E.; AlBunni, H.; Bui, A.D.; Henkel, R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia 2018, 50, e13126. [Google Scholar] [CrossRef]
- Tekin, S.; Erden, Y.; Sandal, S.; Etem Onalan, E.; Ozyalin, F.; Ozen, H.; Yilmaz, B. Effects of apelin on reproductive functions: Relationship with feeding behavior and energy metabolism. Arch. Physiol. Biochem. 2017, 123, 9–15. [Google Scholar] [CrossRef]
- Troisi, A.; Dall’Aglio, C.; Maranesi, M.; Orlandi, R.; Suvieri, C.; Pastore, S.; Bazzano, M.; Martínez-Barbitta, M.; Polisca, A. Presence and localization of apelin and its cognate receptor in canine testes using immunohistochemical and RT-PCR techniques. Vet. Res. Commun. 2023, 47, 937. [Google Scholar] [CrossRef]
- Choubey, M.; Ranjan, A.; Bora, P.S.; Baltazar, F.; Martin, L.J.; Krishna, A. Role of adiponectin as a modulator of testicular function during aging in mice. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 413–427. [Google Scholar] [CrossRef]
- Shultz, T.D.; Bonorden, W.R.; Seaman, W.R. Effect of short-term flaxseed consumption on lignan and sex hormone metabolism in men. Nutr. Res. 1991, 11, 1089–1100. [Google Scholar] [CrossRef]
- Li, W.; Tang, D.; Li, F.; Tian, H.; Yue, X.; Li, F.; Weng, X.; Sun, W.; Wang, W.; Mo, F. Supplementation with dietary linseed oil during peri-puberty stimulates steroidogenesis and testis development in rams. Theriogenology 2017, 102, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Shang, M.; Chen, C.; Chen, Y.; Hua, J.; Sheng, X.; Wang, X.; Xing, K.; Ni, H.; Guo, Y. Dietary supplementation with linseed oil improves semen quality, reproductive hormone, gene and protein expression related to testosterone synthesis in aging layer breeder roosters. Theriogenology 2019, 131, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.C.; Eden, J.A. A review of the clinical effects of phytoestrogens. Obstet. Gynecol. 1996, 87, 897–904. [Google Scholar] [PubMed]
- Martin, P.M.; Horwitz, K.B.; Ryan, D.S.; McGuire, W.L. Phytoestrogen interaction with estrogen receptors in human breast cancer cells. Endocrinology 1978, 103, 1860–1867. [Google Scholar] [CrossRef]
- Apel-Paz, M.; Vanderlick, T.K.; Chandra, N.; Doncel, G.F. A hierarchy of lipid constructs for the sperm plasma membrane. Biochem. Biophys. Res. Commun. 2003, 309, 724–732. [Google Scholar] [CrossRef]
- Nordgren, T.M.; Anderson Berry, A.; Van Ormer, M.; Zoucha, S.; Elliott, E.; Johnson, R.; McGinn, E.; Cave, C.; Rilett, K.; Weishaar, K.; et al. Omega-3 Fatty Acid Supplementation, Pro-Resolving Mediators, and Clinical Outcomes in Maternal-Infant Pairs. Nutrients 2019, 11, 98. [Google Scholar] [CrossRef]
- Zirpoli, H.; Chang, C.L.; Carpentier, Y.A.; Michael-Titus, A.T.; Ten, V.S.; Deckelbaum, R.J. Novel Approaches for Omega-3 Fatty Acid Therapeutics: Chronic Versus Acute Administration to Protect Heart, Brain, and Spinal Cord. Annu. Rev. Nutr. 2020, 40, 161–168. [Google Scholar] [CrossRef]
- Serhan, C.N.; Brain, S.D.; Buckley, C.D.; Gilroy, D.W.; Haslett, C.; O’Neill, L.A.; Perretti, M.; Rossi, A.G.; Wallace, J.L. Resolution of inflammation: State of the art, definitions and terms. FASEB J. 2007, 21, 325. [Google Scholar] [CrossRef]
- Moro, K.; Nagahashi, M.; Ramanathan, R.; Takabe, K.; Wakai, T. Resolvins and omega three polyunsaturated fatty acids: Clinical implications in inflammatory diseases and cancer. World J. Clin. Cases 2016, 4, 155–164. [Google Scholar] [CrossRef]
- Meunier, B.; de Visser, S.P.; Shaik, S. Mechanism of oxidation reactions catalyzed by cytochrome P450 enzymes. Chem. Rev. 2004, 104, 3947–3980. [Google Scholar] [CrossRef]
- Samandari-Bahraseman, M.R.; Elyasi, L. Apelin-13 protects human neuroblastoma SH-SY5Y cells against amyloid-beta induced neurotoxicity: Involvement of anti oxidant and anti apoptotic properties. J. Basic. Clin. Physiol. Pharmacol. 2021, 33, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Chiarella, P.; Puglisi, R.; Sorrentino, V.; Boitani, C.; Stefanini, M. Ryanodine receptors are expressed and functionally active in mouse spermatogenic cells and their inhibition interferes with spermatogonial differentiation. J. Cell Sci. 2004, 117 Pt 18, 4127–4134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ru, Y.; Wang, C.; Wang, S.; Zhou, Z.; Zhang, Y. Tripeptidyl peptidase II regulates sperm function by modulating intracellular Ca2+ stores via the ryanodine receptor. PLoS ONE 2013, 8, e66634. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Signorini, C.; Noto, D.; Corsaro, R.; Micheli, L.; Durand, T.; Oger, C.; Galano, J.M.; Collodel, G. F4-Neuroprostane Effects on Human Sperm. Int. J. Mol. Sci. 2023, 24, 935. [Google Scholar] [CrossRef]
- Dobrev, D.; Heijman, J.; Hiram, R.; Li, N.; Nattel, S. Inflammatory signalling in atrial cardiomyocytes: A novel unifying principle in atrial fibrillation pathophysiology. Nat. Rev. Cardiol. 2023, 20, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, X.; Hjorth, E.; Colas, R.A.; Schroeder, L.; Granholm, A.C.; Serhan, C.N.; Schultzberg, M. Pro-Resolving Lipid Mediators Improve Neuronal Survival and Increase Aβ42 Phagocytosis. Mol. Neurobiol. 2016, 53, 2733–2749. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, Q.; Chen, J.; Yang, Y.; Chen, W.; Mao, H.; Ouyang, X.; Zhang, K.; Tang, M.; Yan, J.; et al. MCU-dependent mitochondrial calcium uptake-induced mitophagy contributes to apelin-13-stimulated VSMCs proliferation. Vascul. Pharmacol. 2022, 144, 106979. [Google Scholar] [CrossRef]
- Boiti, C.; Castellini, C.; Besenfelder, U.; Theau-Clément, M.; Liguori, L.; Renieri, T.; Pizzi, F. Guidelines for the handling of rabbit bucks and semen. World Rabbit. Sci. 2005, 13, 71–91. [Google Scholar]
- Shara, M.A.; Dickson, P.H.; Bagchi, D.; Stohs, S.J. Excretion of formaldehyde, malondialdehyde, acetaldehyde and acetone in the urine of rats in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin, paraquat, endrin and carbon tetrachloride. J. Chromatogr. 1992, 576, 221–233. [Google Scholar] [CrossRef]
- Mourvaki, E.; Cardinali, R.; Dal Bosco, A.; Corazzi, L.; Castellini, C. Effects of flaxseed dietary supplementation on sperm quality and on lipid composition of sperm subfractions and prostatic granules in rabbit. Theriogenology 2010, 73, 629–637. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, H. Asimplemethod for the isolation and purification of total lipids from animal tissue. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W. A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid Res. 1982, 23, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, S.; Dal Bosco, A.; Maranesi, M.; Petrucci, L.; Rebollar, P.G.; Castellini, C. Dietary fish oil and flaxseed for rabbit does: Fatty acids distribution and Δ6-desaturase enzyme expression of different tissues. Animal 2019, 13, 1934–1942. [Google Scholar] [CrossRef] [PubMed]




| Groups | Serum Testosterone (pg/mL) | Sperm Motility (%) | VCL (µm/sec) | MDA (nmol MDA/mL) | n-3 PUFA (% of Total FA) | n-6 PUFA (% of Total FA) | n-3 VLCP (% of Total FA) | n-6 VLCP (% of Total FA) |
|---|---|---|---|---|---|---|---|---|
| Control | 3.63 b | 62.13 a | 184.7 ab | 2.82 a | 0.63 a | 38.56 c | 0.39 a | 26.12 c |
| FLAX | 4.60 c | 76.31 b | 236.5 b | 14.79 b | 4.25 b | 22.45 b | 2.83 b | 18.31 a |
| FISH | 2.82 a | 77.27 b | 226.9 b | 20.64 c | 13.12 c | 19.06 a | 12.62 c | 23.62 b |
| SE | 0.15 | 1.29 | 7.05 | 0.31 | 0.15 | 0.21 | 0.11 | 0.29 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Groups | Apelin (ng/g) | RvD1 (pg/g) | MDA (nmol/g) | n-3 PUFA (%) | n-6 PUFA (%) | n-3 VLCP (%) | n-6 VLCP (%) |
|---|---|---|---|---|---|---|---|
| Control | 1.187 a | 111.578 a | 34.755 a | 2.305 a | 38.695 b | 0.078 a | 26.525 b |
| FLAX | 2.400 b | 382.058 b | 42.295 c | 7.747 c | 32.428 a | 1.602 c | 18.700 a |
| FISH | 1.166 a | 394.157 b | 37.370 b | 4.470 b | 32.262 a | 0.950 bc | 17.930 a |
| SE | 0.197 | 20.971 | 0.270 | 0.192 | 0.312 | 0.036 | 0.325 |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| RvD1 Testis | MDA Testis | n-3 PUFA Testis | n-6 PUFA Testis | T | MDA Sperm | n-3 PUFA Sperm | n-6 PUFA Sperm | VCL | Sperm Motility | n-3 PUFA Intake | n-6 PUFA Intake | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apelin testis | 0.360 | 0.838 ** | 0.798 ** | −0.408 | 0.692 ** | 0.145 | −00.210 | −0.292 | 0.567 * | 0.376 | 0.819 ** | 0.859 ** |
| RvD1 testis | 0.608 ** | 0.736 ** | −0.947 ** | −0.002 | 0.930 ** | 0.721 ** | −0.960 ** | 0.776 ** | 0.949 ** | 0.724 ** | 0.248 | |
| MDA testis | 0.964 ** | −0.639 ** | 0.742 ** | 0.387 | −0.033 | −0.531 * | 0.708 ** | 0.607 ** | 0.980 ** | 0.900 ** | ||
| n-3 PUFA testis | −0.780 ** | 0.593 ** | 0.560 * | 0.168 | −0.684 ** | 0.828 ** | 0.718 ** | 0.990 ** | 0.799 ** | |||
| n-6 PUFA testis | −0.029 | −0.936 ** | −0.730 ** | 0.975 ** | −0.888 ** | −0.945 ** | −0.747 ** | −0.267 | ||||
| T | −0.262 | −0.621 ** | 0.105 | 0.192 | −0.023 | 0.643 ** | 0.925 ** | |||||
| MDA sperm | 0.904 ** | −0.984 ** | 0.777 ** | 0.930 ** | 0.524 * | −0.031 | ||||||
| n-3 PUFA sperm | −0.825 ** | 0.540 * | 0.724 ** | 0.119 | −0.448 | |||||||
| n-6 PUFA sperm | −0.840 ** | −0.956 ** | −0.657 ** | −0.133 | ||||||||
| VCL | 0.815 ** | 0.779 ** | 0.406 | |||||||||
| Sperm motility | 0.704 ** | 0.228 | ||||||||||
| n-3 PUFA intake | 0.406 |
| Control | FLAX | FISH | |
|---|---|---|---|
| Dehydrated alfalfa meal | 300 | 380 | 380 |
| Soybean meal 44% | 150 | 100 | 150 |
| Barley meal | 410 | 310 | 335 |
| Wheat bran | 52 | 52 | 52 |
| Soybean oil | 30 | - | - |
| Extruded flaxseed | - | 100 | - |
| Fish oil | - | - | 35 |
| Beet molasses | 20 | 10 | 10 |
| Calcium carbonate | 7 | 7 | 7 |
| Calcium diphosphate | 13.5 | 13.5 | 13.5 |
| Salt | 7 | 7 | 7 |
| DL-methionine | 0.5 | 0.5 | 0.5 |
| Vitamin-mineral premix † | 10 | 10 | 10 |
| Crude protein | 175 | 174 | 175 |
| Ether extract | 480 | 472 | 425 |
| Crude fiber | 124 | 137 | 130 |
| Ash | 89 | 84 | 90 |
| LA | 50.45 | 22.30 | 20.50 |
| ALA | 11.15 | 45.80 | 18.50 |
| n-6 PUFA | 51.45 | 22.80 | 21.00 |
| n-3 PUFA | 11.35 | 46 | 26.40 |
| n-3 VLCP | - | - | 10.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattioli, S.; Moretti, E.; Castellini, C.; Signorini, C.; Corsaro, R.; Angelucci, E.; Collodel, G. Can Dietary n-3 Polyunsaturated Fatty Acids Affect Apelin and Resolvin in Testis and Sperm of Male Rabbits? Molecules 2023, 28, 6188. https://doi.org/10.3390/molecules28176188
Mattioli S, Moretti E, Castellini C, Signorini C, Corsaro R, Angelucci E, Collodel G. Can Dietary n-3 Polyunsaturated Fatty Acids Affect Apelin and Resolvin in Testis and Sperm of Male Rabbits? Molecules. 2023; 28(17):6188. https://doi.org/10.3390/molecules28176188
Chicago/Turabian StyleMattioli, Simona, Elena Moretti, Cesare Castellini, Cinzia Signorini, Roberta Corsaro, Elisa Angelucci, and Giulia Collodel. 2023. "Can Dietary n-3 Polyunsaturated Fatty Acids Affect Apelin and Resolvin in Testis and Sperm of Male Rabbits?" Molecules 28, no. 17: 6188. https://doi.org/10.3390/molecules28176188
APA StyleMattioli, S., Moretti, E., Castellini, C., Signorini, C., Corsaro, R., Angelucci, E., & Collodel, G. (2023). Can Dietary n-3 Polyunsaturated Fatty Acids Affect Apelin and Resolvin in Testis and Sperm of Male Rabbits? Molecules, 28(17), 6188. https://doi.org/10.3390/molecules28176188