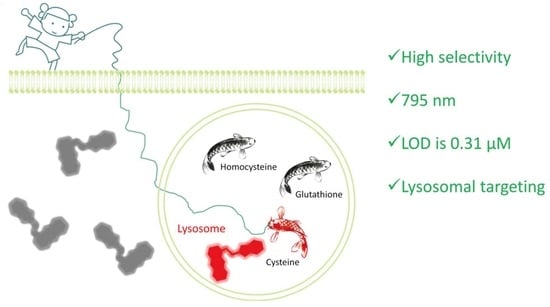
A New Lysosome-Targeted NIR Fluorescent Probe for Specific Detection of Cysteine over Homocysteine and Glutathione
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis
3.2. MTT Assay
3.3. Cell Culture and Fluorescence Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wlodek, P.J.; Iciek, M.B.; Milkowski, A.; Smolenski, O.B. Various forms of plasma cysteine and its metabolites in patients undergoing hemodialysis. Clin. Chim. Acta 2001, 304, 9. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.V.; Templeton, D.J. Regulation of signal transduction through protein cysteine oxidation. Antioxid. Redox Signal. 2006, 8, 1819. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999, 27, 916. [Google Scholar] [CrossRef]
- Hartle, D.M.; Pluth, M.D. A practical guide to working with H2S at the interface of chemistry and biology. Chem. Soc. Rev. 2016, 45, 6108. [Google Scholar] [CrossRef] [PubMed]
- Hultberg, B.; Andersson, A.; Isaksson, A. The cell-damaging effects of low amounts of homocysteine and copper ions in human cell line cultures are caused by oxidative stress. Toxicology 1997, 123, 33. [Google Scholar] [CrossRef]
- Dominy, J.E.; Stipanuk, M.H. New roles for cysteine and transsulfuration enzymes: Production of H2S, a neuromodulator and smooth muscle relaxant. Nutr. Rev. 2004, 62, 348. [Google Scholar] [CrossRef] [PubMed]
- Weerapana, E.; Wang, C.; Simon, G.M.; Richter, F.; Khare, S.; Dillon, M.B.D.; Bachovchin, D.A.; Mowen, K.; Baker, D.; Cravatt, B.F. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 2010, 468, 790. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Carroll, K.S. Cysteine-mediated redox signaling: Chemistry, biology, and tools for discovery. Chem. Rev. 2013, 113, 4633. [Google Scholar] [CrossRef]
- Park, S.; Imlay, J.A. High levels of intracellular cysteine promote oxidative DNA damage by driving the Fenton reaction. J. Bacteriol. 2003, 185, 1942. [Google Scholar] [CrossRef]
- El-Khairy, L.; Ueland, P.M.; Refsum, H.; Graham, I.; Vollset, S.E. Plasma total cysteine as a risk factor for vascular disease. Circulation 2001, 103, 2544. [Google Scholar] [CrossRef]
- Gahl, W.A.; Tietze, F.; Bashan, N.; Steinherz, R.; Schulman, J.D. Defective cystine exodus from isolated lysosome-rich fractions of cystinotic leucocytes. J. Biol. Chem. 1982, 257, 9570. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.B. Disulphide reduction in lysosomes, the role of cysteine. Biochem. J. 1986, 237, 271. [Google Scholar] [CrossRef] [PubMed]
- Peake, R.G.; Balasubramanian, K.L.; Diess, W.P., Jr. Effect of reduced glutathione on the proteolysis of intra-particulate and native thyroglobulin. Biochim. Biophys. Acta 1967, 148, 689. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Peng, X.; Yoon, J. Fluorescent and colorimetric probes for detection of thiols. Chem. Soc. Rev. 2010, 39, 2120. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.; Huo, Y.; Zhang, H.; Wang, L.; Zhang, P.; Song, D.; Shi, Y.; Guo, W. Simultaneous fluorescence sensing of Cys and GSH from different emission channels. J. Am. Chem. Soc. 2014, 136, 574. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Tong, J.; Wang, J.; Qin, A.; Sun, J.; Tang, B. Discriminative fluorescence detection of cysteine, homocysteine and glutathione via reaction-dependent aggregation of fluorophore-analyte adducts. J. Mater. Chem. 2012, 22, 17063. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Q.; Huang, J.; Li, N.; Gu, Y. A dual-site fluorescent probe for direct and highly selective detection of cysteine and its application in living cells. Biosens. Bioelectron. 2017, 92, 583. [Google Scholar] [CrossRef]
- Cao, X.; Cao, W.; Lin, W.; Yu, Q. A ratiometric fluorescent probe for thiols based on a tetrakis (4-hydroxyphenyl) porphyrin–coumarin scaffold. J. Org. Chem. 2011, 76, 7423. [Google Scholar] [CrossRef]
- Chen, F.; Han, D.; Gao, Y.; Liu, H.; Wang, S.; Zhou, F.; Li, K.; Zhang, S.; Shao, W.; He, Y. A turn-on fluorescent probe for simultaneous sensing of cysteine/homocysteine and hydrogen sulfide and its bioimaging applications. Talanta 2018, 187, 19. [Google Scholar] [CrossRef]
- Long, Z.; Chen, L.; Dang, Y.; Chen, D.; Lou, X.; Xia, F. An ultralow concentration of two-photon fluorescent probe for rapid and selective detection of lysosomal cysteine in living cells. Talanta 2019, 204, 762. [Google Scholar] [CrossRef]
- Qi, Y.; Huang, Y.; Li, B.; Zeng, F.; Wu, S. Real-time monitoring of endogenous cysteine levels in vivo by near-infrared turn-on fluorescent probe with large stokes shift. Anal. Chem. 2018, 90, 1014. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, C.; Jiao, X.; Cai, S.; He, S.; Zhao, L.; Zeng, X.; Wang, T. Lysosome-targeted near-infrared fluorescent dye and its application in designing of probe for sensitive detection of cysteine in living cells. Dye. Pigment. 2021, 190, 109293. [Google Scholar] [CrossRef]
- Weissleder, R.; Ntziachristos, V. Shedding light onto live molecular targets. Nat. Med. 2003, 9, 123. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, C.; Cai, S.; He, S.; Zhao, L.; Zeng, X.; Gong, J. A highly sensitive sensor for colorimetric detection of palladium(II) in lysosomes and its applications. Dalton Trans. 2022, 51, 3116. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Jiao, X.; Liu, C.; Zheng, D.; Huang, K.; Wang, Q.; He, S.; Zhao, L.; Zeng, X. A selective and sensitive fluorescent probe for homocysteine and its application in living cells. Dye. Pigment. 2017, 140, 212. [Google Scholar] [CrossRef]
- Gong, J.; Liu, C.; Jiao, X.; He, S.; Zhao, L.; Zeng, X. A novel near-infrared fluorescent probe with large stokes shifts for sensing extreme acidity and its application in bioimaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 243, 118821. [Google Scholar] [CrossRef]
- Ge, C.; Shen, F.; Yin, Y.; Chang, K.; Zhang, X.; Zhou, P.; Li, J.; Liu, Y.; Lu, C. A novel NIR fluorescence probe with cysteine-activated structure for specific detection of cysteine and its application in vitro and in vivo. Talanta 2021, 223, 121758. [Google Scholar] [CrossRef]
- Ye, Z.; Duan, C.; Hu, Q.; Zhang, Y.; Qin, C.; Zeng, L. A dual-channel responsive near-infrared fluorescent probe for multicolour imaging of cysteine in living cells. J. Mater. Chem. B 2017, 5, 3600. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Wu, X.; Liu, B.; Zhang, J. Discriminative detection of cysteine/homocysteine and glutathione in HeLa cells by dual-channel fluorescent probe. Dye. Pigment. 2021, 186, 109015. [Google Scholar] [CrossRef]
- Song, X.; Jing, C.; Wang, Y.; Feng, Y.; Cao, C.; Wang, K.; Liu, W.; Ru, J. Fluorescence distinguishing of SO2 derivatives and Cys/GSH from multi-channel signal patterns and visual sensing based on smartphone in living cells and environment. J. Hazard. Mater. 2021, 413, 125332. [Google Scholar] [CrossRef]
- Cao, C.; Jing, Y.; Feng, X.; Song, W.; Liu, G.; Zhang, G.; Dou, W.; Ru, J. A novel bis-reaction-triggered cascade fluorescent probe for improved specific detection and biological visualization of Cys over Hcy/GSH. Dye. Pigment. 2022, 197, 109823. [Google Scholar] [CrossRef]
- Sun, Y.; Han, H.; Huang, J.; Li, J.; Zang, Y.; Wang, C. A long-wavelength fluorescent probe with a large Stokes shift for lysosome-targeted imaging of Cys and GSH. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 261, 120055. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhuo, S.; Tang, H.; Cao, D. An efficient fluorescent probe for rapid sensing of different concentration ranges of cysteine with two-stage ratiometric signals. Dye. Pigment. 2018, 157, 284. [Google Scholar] [CrossRef]
- Sheng, H.; Hu, Y.; Zhou, Y.; Fan, S.; Cao, Y.; Zhao, X.; Yang, W. A highly selective ESIPT-based fluorescent probe with a large Stokes shift for the turn-on detection of cysteine and its application in living cells. Dye. Pigment. 2019, 160, 48. [Google Scholar] [CrossRef]
- Xiong, K.; Huo, F.; Chao, J.; Zhang, Y.; Yin, C. Colorimetric and NIR fluorescence probe with multiple binding sites for distinguishing detection of Cys/Hcy and GSH in vivo. Anal. Chem. 2019, 91, 1472. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, D.; Wu, J.; Xia, Q.; Jia, X.; Song, X.; Zeng, L.; Yuan, Y. A water-soluble near-infrared fluorescent probe for sensitive and selective detection of cysteine. Talanta 2019, 204, 747. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Liu, C.; He, S.; Zeng, X.; Zhang, J.; Gong, J. A New Lysosome-Targeted NIR Fluorescent Probe for Specific Detection of Cysteine over Homocysteine and Glutathione. Molecules 2023, 28, 6189. https://doi.org/10.3390/molecules28176189
Liu Q, Liu C, He S, Zeng X, Zhang J, Gong J. A New Lysosome-Targeted NIR Fluorescent Probe for Specific Detection of Cysteine over Homocysteine and Glutathione. Molecules. 2023; 28(17):6189. https://doi.org/10.3390/molecules28176189
Chicago/Turabian StyleLiu, Qiuchen, Chang Liu, Song He, Xianshun Zeng, Jian Zhang, and Jin Gong. 2023. "A New Lysosome-Targeted NIR Fluorescent Probe for Specific Detection of Cysteine over Homocysteine and Glutathione" Molecules 28, no. 17: 6189. https://doi.org/10.3390/molecules28176189
APA StyleLiu, Q., Liu, C., He, S., Zeng, X., Zhang, J., & Gong, J. (2023). A New Lysosome-Targeted NIR Fluorescent Probe for Specific Detection of Cysteine over Homocysteine and Glutathione. Molecules, 28(17), 6189. https://doi.org/10.3390/molecules28176189