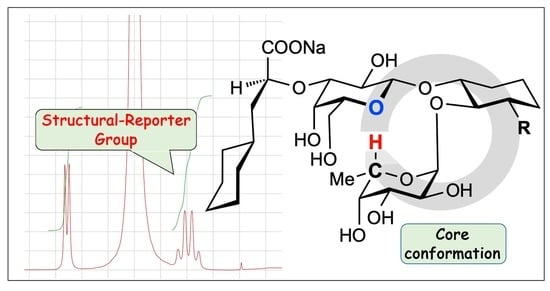
A Structural-Reporter Group to Determine the Core Conformation of Sialyl Lewisx Mimetics
Abstract
:1. Introduction
2. Results
2.1. Synthesis of 3-Alkyl/aryl-2-cyclohexen-1-ols (3b-m)
2.2. Enzyme-Catalyzed Kinetic Resolution of Rac-3-alkyl/aryl-2-cyclohexen-1-ol (3b-n)
2.3. Synthesis of the Mimetics 2b-n of Sialyl Lewisx (1)
2.4. Structural-Reporter Group for the Core Conformation
3. Discussion
 | |||||||||
| Comp. | R | KD [μM] | H-C5Fuc δ [ppm] | Comp. | R | KD [μM] | H-C5Fuc δ [ppm] | ||
| 2a | H | 60.7 | 4.50 [17] | 2h | t-Bu | 51 | 4.03 | ||
| 2b | Me | 17.8 | 4.84 [17] | 2i | n-Bu | 5.4 | 4.86 | ||
| 2c | Et | 9.5 | 4.84 [17] | 2j | n-Hex | 4.3 | 4.83 | ||
| 2d | i-Pr | 6.8 | 4.85 | 2k | (CH2)3Ph | 28 | 4.75 | ||
| 2e | Benzyl | 18 | 4.87 | 2l | CH2O(CH2)2OMe | 31 | 4.84 | ||
| 2f | CH2C6H11 | 14 | 4.83 | 2m | i-Bu | 6 | 4.85 | ||
| 2g | Phenyl | 8.8 | 4.75 | 2n | CH2CF3 | 9.5 | 4.61 | ||
| Reference compounds | |||||||||
| Comp. | KD [μM] | H-C5Fuc δ [ppm] | |||||||
| 20 | 3.93 [33] | ||||||||
| 21 | 4922 | 4.12 [23] | |||||||
| 22 | 280 [11,12,13] | 4.83 [17] | |||||||
| sLex (1) | 877 [7] | 4.83 [32] | |||||||
4. Conclusions
5. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ernst, B.; Magnani, J.L. From carbohydrate leads to glycomimetic drugs. Nat. Rev. Drug Discov. 2009, 8, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Zetterberg, F.R.; MacKinnon, A.; Brimert, T.; Gravelle, L.; Johnsson, R.E.; Kahl-Knutson, B.; Leffler, H.; Nilsson, U.J.; Pedersen, A.; Peterson, K.; et al. Discovery and optimization of the first highly effective and orally available galectin-3 inhibitor for the treatment of fibrotic disease. J. Med. Chem. 2022, 65, 12626–12638. [Google Scholar] [CrossRef] [PubMed]
- Slack, R.J.; Mills, R.; Mackinnon, A.C. The therapeutic potential of galectin-3 inhibition in fibrotic disease. Int. J. Biochem. Cell Biol. 2021, 130, 105881. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Sari-Ak, D.; Bagga, T. Siglecs as therapeutic targets in cancer. Biology 2021, 10, 1178–1205. [Google Scholar] [CrossRef] [PubMed]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007, 7, 255–266. [Google Scholar] [CrossRef]
- Kim, C.U.; Lew, W.; Williams, M.A.; Liu, H.T.; Zhang, L.J.; Swaminathan, S.; Bischofberger, N.; Chen, M.S.; Mendel, D.B.; Tai, C.Y.; et al. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site. J. Am. Chem. Soc. 1997, 119, 681–690. [Google Scholar] [CrossRef]
- Binder, F.P.C.; Lemme, K.; Preston, R.C.; Ernst, B. Sialyl Lewisx: A “pre-organized water oligomer”. Angew. Chem. Int. Ed. 2012, 51, 73277331. [Google Scholar] [CrossRef]
- Cabani, S.; Gianni, P.; Mollica, V.; Lepori, L. Group contributions to the thermodynamic properties of non-ionic organic solutes in dilute aqueous solution. J. Solution Chem. 1981, 10, 563–595. [Google Scholar] [CrossRef]
- Vedani, A.; Huhta, D.W. A new force field for modeling metalloproteins. J. Am. Chem. Soc. 1990, 112, 4759–4767. [Google Scholar] [CrossRef]
- D’Aquino, J.A.; Freire, E.; Amzel, L.M. Binding of small organic molecules to macromolecular targets: Evaluation of conformational entropy charges. Proteins 2000, 4, 93–107. [Google Scholar] [CrossRef]
- Kolb, H.C.; Ernst, B. Development of tools for the design of selectin antagonists. Chem. Eur. J. 1997, 3, 1571–1578. [Google Scholar] [CrossRef]
- Jahnke, W.; Kolb, H.C.; Blommers, M.J.J.; Magnani, J.L.; Ernst, B. Comparison of the bioactive conformation of sialyl Lewisx and a potent sialyl Lewisx mimic. Angew. Chem. Int. Ed. Engl. 1997, 36, 2603–2607. [Google Scholar] [CrossRef]
- Norman, K.E.; Anderson, G.P.; Kolb, H.C.; Ley, K.; Ernst, B. Sialyl Lewisx (sLex) and an sLex mimetic, CGP69669A, disrupt E-selectin-dependent leukocyte rolling in vivo. Blood 1998, 91, 475–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holub, N.; Neidhöfer, J.; Blechert, S. The total synthesis of (+)-trans-195A. Org. Lett. 2005, 7, 1227–1229. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J.; Helal, C.J. Reduction of carbonyl compounds with chiral oxazaborolidine catalysts: A new paradigm for enantioselective catalysis and a powerful new synthetic method. Angew. Chem. Int. Ed. 1998, 37, 1986–2012. [Google Scholar] [CrossRef]
- Alexakis, A.; Jachiet, D.; Normand, J.F. Boron fluoride promoted opening of epoxides by organocopper and cuprate reagents. Tetrahedron 1986, 42, 5607–5619. [Google Scholar] [CrossRef]
- Schwizer, D.; Patton, J.T.; Cutting, B.; Smieško, M.; Wagner, B.; Kato, A.; Weckerle, C.; Binder, F.P.; Rabbani, S.; Schwardt, O.; et al. Pre-organization of the core structure of E-selectin antagonists. Chem. Eur. J. 2012, 18, 1342–1351. [Google Scholar] [CrossRef]
- Van Leeuwen, S.S.; Leeflang, B.R.; Gerwig, G.J.; Kamerling, J.P. Development of a 1H NMR structural-reporter group concept for the primary structural characterization of α-d-glucans. Carbohydr. Res. 2008, 343, 1114–1119. [Google Scholar] [CrossRef] [Green Version]
- Spik, G.; Debruyne, V.; Montreuil, J.; van Halbeck, H.; Vliegenthart, J.F.G. Primary structures of two sialylated triantennary glycans from human serotransferrin. FEBS 1985, 183, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Zierke, M.; Smieško, M.; Rabbani, S.; Aeschbacher, T.; Cutting, B.; Allain, F.H.-T.; Schubert, M.; Ernst, B. Stabilization of branched oligosaccharides: Lewisx benefits from a non-conventional C-H•••O hydrogen bond. J. Am. Chem. Soc. 2013, 135, 13464–13472. [Google Scholar] [CrossRef]
- Aeschbacher, T.; Zierke, M.; Smieško, M.; Collot, M.; Mallet, J.-M.; Ernst, B.; Allain, F.H.-T.; Schubert, M. A secondary structural element in a wide range of fucosylated glycoepitopes. Chem. Eur. J. 2017, 23, 11598–11610. [Google Scholar] [CrossRef] [PubMed]
- Battistell, M.D.; Azurmendi, H.F.; Frank, M.; Freedberg, D.I. Uncovering nonconventional and conventional hydrogen bonds in oligosaccharides through NMR experiments and molecular modeling: Application to sialyl Lewisx. J. Am. Chem. Soc. 2015, 137, 13444–13447. [Google Scholar] [CrossRef]
- Rotticci, D.; Norin, T.; Hult, K. Mass transport limitation reduce the effective stereospecificity in enzyme-catalyzed kinetic resolution. Org. Lett. 2000, 2, 1373–1376. [Google Scholar] [CrossRef]
- Ter Halle, R.; Bernet, Y.; Billard, S.; Bufferne, C.; Carlier, P.; Delaitre, C.; Flouzat, C.; Humbolt, G.; Laigle, J.C.; Lombard, F.; et al. Development of a practical multikilogram production of (R)-seudenol by enzymatic resolution. Org. Process Res. Dev. 2004, 8, 283–286. [Google Scholar] [CrossRef]
- Barnier, J.-P.; Morisson, V.; Volle, J.; Blanco, L. Chemo-enzymatic preparation of optically active endo-bicyclo [4.1.0]heptan-2-ols. Tetrahedron Asymmetry 1999, 10, 1107–1117. [Google Scholar] [CrossRef]
- Luche, J.-L. Lanthanides in organic chemistry. 1. Selective 1,2 reduction of conjugated ketones. J. Am. Chem. Soc. 1978, 100, 2226–2227. [Google Scholar] [CrossRef]
- Li, J.; Tan, C.; Gong, J.; Yang, Z. Palladium-catalyzed oxidative rearrangement of tertiary allylic alcohols to enones with oxygen in aqueous solvent. Org. Lett. 2014, 16, 5370–5373. [Google Scholar] [CrossRef]
- Munyemana, F.; Frique-Hesbain, A.-M.; Devos, A.; Ghosez, L. Synthesis of alkyl halides under neutral conditions. Tetrahedron Lett. 1989, 30, 3077–3080. [Google Scholar] [CrossRef]
- Chen, Q.-Y.; Wu, S.-W. Methyl fluorosulphonyldifluoroacetate; a new trifluoromethylating agent. J. Chem. Soc. Chem. Commun. 1989, 705–706. [Google Scholar] [CrossRef]
- Sato, S.; Mori, M.; Ito, Y.; Ogawa, T. An efficient approach to O-glycosides through CuBr2—Bu4NBr mediated activation of glycosides. Carbohydr. Res. 1986, 155, C6–C10. [Google Scholar] [CrossRef]
- Ernst, B.; Wagner, B.; Baisch, G.; Katopodis, A.; Winkler, T.; Öhrlein, R. Substrate specificity of fucosyl transferase III: An efficient synthesis of sialyl Lewisx-, sialyl Lewisa-derivatives and mimetics thereof. Can. J. Chem. 2000, 78, 892–904. [Google Scholar] [CrossRef]
- Ball, G.E.; O’Neill, R.A.; Schultz, J.E.; Lowe, J.B.; Weston, B.W.; Nagy, J.O.; Brown, E.G.; Hobbs, C.J.; Bednarski, M.D. Synthesis and structural analysis using 2-D NMR of sialyl Lewisx (sLex) and Lewisx (Lex) oligosaccharides: Ligands related to E-selectin [ELAM-1] binding. J. Am. Chem. Soc. 1992, 114, 5449–5451. [Google Scholar] [CrossRef]
- Zehavi, U.; Sharon, N. The synthesis of methyl 2,4-diacetamido-2,4,6-trideoxy hexapyranosides. J. Org. Chem. 1972, 37, 2141–2145. [Google Scholar] [CrossRef] [PubMed]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- Thoma, G.; Magnani, J.L.; Patton, J.T.; Ernst, B.; Jahnke, W. Preorganization of the bioactive conformation of sialyl Lewisx analogues correlates with their affinity to E-selectin. Angew. Chem. Int. Ed. 2001, 40, 1941–1945. [Google Scholar] [CrossRef]
- Preston, R.C.; Jakob, R.J.; Binder, F.P.C.; Sager, C.P.; Ernst, B.; Maier, T. E-selectin ligand complexes adopt an extended high-affinity conformation. J. Mol. Cell. Biol. 2016, 8, 62–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, A. Thermodynamics and Interaction. In RSC Tutorial Chemistry Text No. 16. Biophysical Chemistry; Humphrey, S., Macpherson, J., Taylor, P., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2004; pp. 109–110. [Google Scholar]






| Comp. | R | de [%] | Comp. | R | de [%] |
|---|---|---|---|---|---|
| 12b | Me | 97 | 12i | n-Bu | 90 |
| 12c | Et | 92 | 12j | n-Hex | 94 |
| 12d | i-Pr | 85 | 12k | (CH2)3Ph | 98 |
| 12e | Benzyl | 97 | 12l | CH2O(CH2)2OMe | 98 |
| 12f | CH2C6H11 | 97 | 12m | i-Bu | 96 |
| 12g | Phenyl | 97 | 12n | CH2CF3 | 96 |
| 12h | t-Bu | 89 |
| Comp. | KD [μM] | H-C5Fuc δ [ppm] | ΔG [kJ mol−1] (Gibbs Free Energy) |
|---|---|---|---|
| 21 | 4922 | 4.12 | −13.17 |
| 2a | 60.7 | 4.50 | −24.06 |
| 2b | 17.8 | 4.84 | −27.12 |
| 2j | 4.3 | 4.83 | −30.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, B.; Binder, F.P.C.; Jiang, X.; Mühlethaler, T.; Preston, R.C.; Rabbani, S.; Smieško, M.; Schwardt, O.; Ernst, B. A Structural-Reporter Group to Determine the Core Conformation of Sialyl Lewisx Mimetics. Molecules 2023, 28, 2595. https://doi.org/10.3390/molecules28062595
Wagner B, Binder FPC, Jiang X, Mühlethaler T, Preston RC, Rabbani S, Smieško M, Schwardt O, Ernst B. A Structural-Reporter Group to Determine the Core Conformation of Sialyl Lewisx Mimetics. Molecules. 2023; 28(6):2595. https://doi.org/10.3390/molecules28062595
Chicago/Turabian StyleWagner, Beatrice, Florian P. C. Binder, Xiaohua Jiang, Tobias Mühlethaler, Roland C. Preston, Said Rabbani, Martin Smieško, Oliver Schwardt, and Beat Ernst. 2023. "A Structural-Reporter Group to Determine the Core Conformation of Sialyl Lewisx Mimetics" Molecules 28, no. 6: 2595. https://doi.org/10.3390/molecules28062595
APA StyleWagner, B., Binder, F. P. C., Jiang, X., Mühlethaler, T., Preston, R. C., Rabbani, S., Smieško, M., Schwardt, O., & Ernst, B. (2023). A Structural-Reporter Group to Determine the Core Conformation of Sialyl Lewisx Mimetics. Molecules, 28(6), 2595. https://doi.org/10.3390/molecules28062595