Development of Type 1 Diabetes in Mice Is Associated with a Decrease in IL-2-Producing ILC3 and FoxP3+ Treg in the Small Intestine
Abstract
:1. Introduction
2. Results
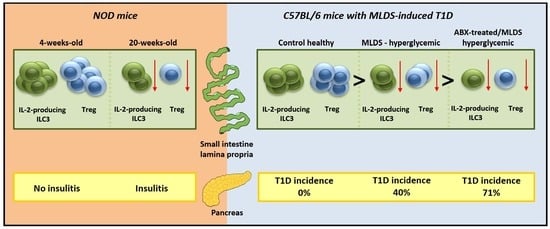
2.1. SILP ILC3 and Treg in NOD Mice
2.2. ILC3 and Treg in SILP of C57BL/6 Mice with MLDS-Induced Diabetes
2.3. The Effect of Antibiotics on SILP ILC3 and Treg in MLDS-Induced Diabetes
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. T1D Induction and Treatment
4.3. Antibiotic Treatment
4.4. Cell Isolation from SILP
4.5. Cell Isolation from Spleen and PLN
4.6. Cell Isolation from the Pancreatic Infiltrates
4.7. Flow cytometric Analysis
4.8. ELISA
4.9. Histology
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Lehuen, A.; Diana, J.; Zaccone, P.; Cooke, A. Immune cell crosstalk in type 1 diabetes. Nat. Rev. Immunol. 2010, 10, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, K.T.; Dotta, F.; Amirian, N.; Campbell, P.D.; Kay, T.W.H.; Atkinson, M.A.; Roep, B.O.; von Herrath, M.G. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J. Exp. Med. 2012, 209, 51–60. [Google Scholar] [CrossRef]
- Yu, H.; Paiva, R.; Flavell, R.A. Harnessing the power of regulatory T-cells to control autoimmune diabetes: Overview and perspective. Immunology 2018, 153, 161–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, J.C.; Clayton, D.G.; Concannon, P.; Akolkar, B.; Cooper, J.D.; Erlich, H.A.; Julier, C.; Morahan, G.; Nerup, J.; Nierras, C.; et al. Type 1 Diabetes Genetics Consortium, Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat. Genet. 2009, 41, 703–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mejía-León, M.E.; Calderón de la Barca, A.M. Diet, Microbiota and Immune System in Type 1 Diabetes Development and Evolution. Nutrients 2015, 7, 9171–9184. [Google Scholar] [CrossRef] [Green Version]
- Wells, J.M.; Loonen, L.M.P.; Karczewski, J.M. The role of innate signaling in the homeostasis of tolerance and immunity in the intestine. Int. J. Med. Microbiol. 2009, 300, 41–48. [Google Scholar] [CrossRef]
- Vaarala, O. Is the origin of type 1 diabetes in the gut? Immunol. Cell. Biol. 2012, 90, 271–276. [Google Scholar] [CrossRef]
- Graves, C.L.; Li, J.; LaPato, M.; Shapiro, M.R.; Glover, S.C.; Wallet, M.A.; Wallet, S.M. Intestinal epithelial cell regulation of adaptive immune dysfunction in human Type 1 diabetes. Front. Immunol. 2017, 7, 679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, N.; Peng, J.; Liu, F.; Gulden, E.; Hu, Y.; Zhang, X.; Chen, L.; Wong, F.S.; Wen, L. Microbial antigen mimics activate diabetogenic CD8 T cells in NOD mice. J. Exp. Med. 2016, 213, 2129–2146. [Google Scholar] [CrossRef] [Green Version]
- Pearson, J.A.; Kakabadse, D.; Davies, J.; Peng, J.; Warden-Smith, J.; Cuff, S.; Lewis, M.; da Rosa, L.C.; Wen, L.; Wong, F.S. Altered Gut Microbiota Activate and Expand Insulin B15-23-Reactive CD8+ T Cells. Diabetes 2019, 68, 1002–1013. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Atkinson, M.A. The role for gut permeability in the pathogenesis of type 1 diabetes—A solid or leaky concept? Pediatr. Diabetes 2015, 16, 485–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Đedović, N.; Paunović, V.; Stojanović, I. Isolation and enrichment of mouse insulin-specific CD4+ T regulatory cells. J. Immunol. Methods 2019, 470, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Sorini, C.; Cosorich, I.; Lo Conte, M.; De Giorgi, L.; Facciotti, F.; Lucianò, R.; Rocchi, M.; Ferrarese, R.; Sanvito, F.; Canducci, F.; et al. Loss of gut barrier integrity triggers activation of islet-reactive T cells and autoimmune diabetes. Proc. Natl. Acad. Sci. USA 2019, 116, 15140–15149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, M.C.G.; Oliveira, R.P.; Torres, L.; Aguiar, S.L.F.; Pinheiro-Rosa, N.; Lemos, L.; Guimarães, M.A.; Reis, D.; Silveira, T.; Ferreira, Ê.; et al. Frontline Science: Abnormalities in the gut mucosa of non-obese diabetic mice precede the onset of type 1 diabetes. J. Leukoc. Biol. 2019, 106, 513–529. [Google Scholar] [CrossRef]
- Artis, D.; Spits, D.H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef]
- Hepworth, M.R.; Sonnenberg, G.F. Regulation of the adaptive immune system by innate lymphoid cells. Curr. Opin. Immunol. 2014, 27, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Chu, C.; Teng, F.; Bessman, N.J.; Goc, J.; Santosa, E.K.; Putzel, G.G.; Kabata, H.; Kelsen, J.R.; Baldassano, R.N.; et al. Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature 2019, 568, 405–409. [Google Scholar] [CrossRef]
- Schuster, C.; Jonas, F.; Zhao, F.; Kissler, S. Peripherally induced regulatory T cells contribute to the control of autoimmune diabetes in the NOD mouse model. Eur. J. Immunol. 2018, 48, 1211–1216. [Google Scholar] [CrossRef] [Green Version]
- Godoy, G.J.; Paira, D.A.; Olivera, C.; Breser, M.L.; Sanchez, L.R.; Motrich, R.D.; Rivero, V.E. Differences in T regulatory cells between mouse strains frequently used in immunological re-search: Treg cell quantities and subpopulations in NOD, B6 and BALB/c mice. Immunol. Lett. 2020, 223, 17–25. [Google Scholar] [CrossRef]
- Vecchione, A.; Di Fonte, R.; Gerosa, J.; Jofra, T.; Cicalese, M.P.; Napoleone, V.; Ippolito, E.; Galvani, G.; Ragogna, F.; Stabilini, A.; et al. Reduced PD-1 expression on circulating fol-licular and conventional FOXP3+ Treg cells in children with new onset type 1 diabetes and autoantibody-positive at-risk children. Clin. Immunol. 2020, 211, 108319. [Google Scholar] [CrossRef]
- Yu, H.; Gagliani, N.; Ishigame, H.; Huber, S.; Zhu, S.; Esplugues, E.; Herold, K.C.; Wen, L.; Flavell, R.A. Intestinal type 1 regulatory T cells migrate to periphery to suppress diabetogenic T cells and prevent diabetes development. Proc. Natl. Acad. Sci. USA 2017, 114, 10443–10448. [Google Scholar] [CrossRef] [Green Version]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrick, M.; Smith, M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Mariño, E.; Richards, J.L.; McLeod, K.H.; Stanley, D.; Yap, Y.A.; Knight, J.; McKenzie, C.; Kranich, J.; Oliveira, A.C.; Rossello, F.J.; et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 2017, 18, 552–562. [Google Scholar] [CrossRef]
- Alam, C.; Bittoun, E.; Bhagwat, D.; Valkonen, S.; Saari, A.; Jaakkola, U.; Eerola, E.; Huovinen, P.; Hänninen, A. Effects of a germ-free environment on gut immune regulation and diabetes progression in non-obese diabetic (NOD) mice. Diabetologia 2011, 54, 1398–1406. [Google Scholar] [CrossRef]
- Livanos, A.E.; Greiner, T.U.; Vangay, P.; Pathmasiri, W.; Stewart, D.; McRitchie, S.; Li, H.; Chung, J.; Sohn, J.; Kim, S.; et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat. Microbiol. 2016, 1, 16140. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Tan, W.L.; Soo, C.; Cheung, C.C.; Stewart, J.; Reddy, S. An immunohistochemical study on the distribution and frequency of T regulatory cells in pancreatic islets of NOD mice during various stages of spontaneous and cyclophosphamide-accelerated diabetes. Pancreas 2010, 39, 1024–1033. [Google Scholar] [CrossRef]
- Nti, B.K.; Markman, J.; Bertera, S.; Styche, A.J.; Lakomy, R.J.; Subbotin, V.M.; Trucco, M.; Zorina, T.D. Treg cells in pancreatic lymph nodes: The possible role in diabetogenesis and β cell regeneration in a T1D model. Cell Mol. Immunol. 2012, 9, 455–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candon, S.; Perez-Arroyo, A.; Marquet, C.; Valette, F.; Foray, A.P.; Pelletier, B.; Milani, C.; Ventura, M.; Bach, J.F.; Chatenoud, L. Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes. PLoS ONE 2015, 10, e0125448. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Peng, J.; Tai, N.; Hu, C.; Zhang, X.; Wong, F.S.; Wen, L. Maternal antibiotic treatment protects offspring from diabetes development in nonobese diabetic mice by generation of tolerogenic APCs. J. Immunol. 2015, 195, 4176–4184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, K.; Godovannyi, A.; Ma, C.; Zhang, Y.; Ahmadi-Vand, Z.; Dai, C.; Gorzelak, M.A.; Chan, Y.; Chan, J.M.; Lochner, A.; et al. Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in NOD mice. ISME J. 2016, 10, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.H.F.; Krych, L.; Nielsen, D.S.; Vogensen, F.K.; Hansen, L.H.; Sørensen, S.J.; Buschard, K.; Hansen, A.K. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia 2012, 55, 2285–2294. [Google Scholar] [CrossRef] [Green Version]
- Costa, F.R.C.; Françozo, M.C.S.; de Oliveira, G.G.; Ignacio, A.; Castoldi, A.; Zamboni, D.S.; Ramos, S.G.; Câmara, N.O.; de Zoete, M.R.; Palm, N.W.; et al. Gut microbiota translocation to the pancreatic lymph nodes triggers NOD2 activation and contributes to T1D onset. J. Exp. Med. 2016, 213, 1223–1239. [Google Scholar] [CrossRef] [Green Version]
- Hulme, M.A.; Wasserfall, C.H.; Atkinson, M.A.; Brusko, T.M. Central role for interleukin-2 in type 1 diabetes. Diabetes 2012, 61, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Dogan, Y.; Akarsu, S.; Ustundag, B.; Yilmaz, E.; Gurgoze, M.K. Serum IL-1beta, IL-2, and IL-6 in insulin-dependent diabetic children. Mediators Inflamm. 2006, 2006, 59206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miani, M.; Naour, J.; Waeckel-Enee, E.; chand Verma, S.; Straube, M.; Emond, P.; Ryffel, B.; van Endert, P.; Sokol, H.; Diana, J. Gut Microbiota-Stimulated Innate Lymphoid Cells Support β-Defensin 14 Expression in Pancreatic Endocrine Cells, Preventing Autoimmune Diabetes. Cell Metab. 2018, 28, 557–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maclaren, N.K.; Neufeld, M.J.; McLaughlin, V.; Taylor, G. Androgen sensitization of streptozotocin-induced diabetes in mice. Diabetes 1980, 29, 710–716. [Google Scholar] [CrossRef]
- Paik, S.G.; Michelis, M.A.; Kim, Y.T.; Shin, S. Induction of insulin-dependent diabetes by streptozotocin. Inhibition by estrogens and potentiation by androgens. Diabetes 1982, 31, 724–729. [Google Scholar] [CrossRef]
- Koprivica, I.; Gajic, D.; Saksida, T.; Cavalli, E.; Auci, D.; Despotovic, S.; Pejnovic, N.; Stosic-Grujicic, S.; Nicoletti, F.; Stojanovic, I. Orally delivered all-trans-retinoic acid- and transforming growth factor-β-loaded microparticles ameliorate type 1 diabetes in mice. Eur. J. Pharmacol. 2019, 864, 172721. [Google Scholar] [CrossRef]
- Weigmann, B.; Tubbe, I.; Seidel, D.; Nicolaev, A.; Becker, C.; Neurath, M.F. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat. Protoc. 2007, 2, 2307–2311. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saksida, T.; Paunović, V.; Koprivica, I.; Mićanović, D.; Jevtić, B.; Jonić, N.; Stojanović, I.; Pejnović, N. Development of Type 1 Diabetes in Mice Is Associated with a Decrease in IL-2-Producing ILC3 and FoxP3+ Treg in the Small Intestine. Molecules 2023, 28, 3366. https://doi.org/10.3390/molecules28083366
Saksida T, Paunović V, Koprivica I, Mićanović D, Jevtić B, Jonić N, Stojanović I, Pejnović N. Development of Type 1 Diabetes in Mice Is Associated with a Decrease in IL-2-Producing ILC3 and FoxP3+ Treg in the Small Intestine. Molecules. 2023; 28(8):3366. https://doi.org/10.3390/molecules28083366
Chicago/Turabian StyleSaksida, Tamara, Verica Paunović, Ivan Koprivica, Dragica Mićanović, Bojan Jevtić, Natalija Jonić, Ivana Stojanović, and Nada Pejnović. 2023. "Development of Type 1 Diabetes in Mice Is Associated with a Decrease in IL-2-Producing ILC3 and FoxP3+ Treg in the Small Intestine" Molecules 28, no. 8: 3366. https://doi.org/10.3390/molecules28083366
APA StyleSaksida, T., Paunović, V., Koprivica, I., Mićanović, D., Jevtić, B., Jonić, N., Stojanović, I., & Pejnović, N. (2023). Development of Type 1 Diabetes in Mice Is Associated with a Decrease in IL-2-Producing ILC3 and FoxP3+ Treg in the Small Intestine. Molecules, 28(8), 3366. https://doi.org/10.3390/molecules28083366