One-Pot Exfoliation of Graphite and Synthesis of Nanographene/Dimesitylporphyrin Hybrids
Abstract
:1. Introduction
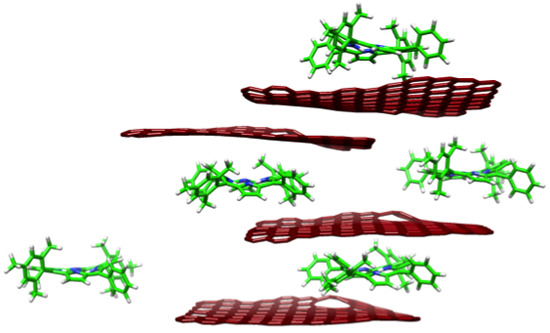
2. Results and Discussion






| Sample | FWHM (cm−1) | 2D/G |
|---|---|---|
| Graphite | 89 | 0.25 |
| FLG | 86 | 0.82 |
| FLG/DMPP | 78 | 0.74 |
| FLG/ZnDMPP | 75 | 0.66 |

3. Experimental Section
3.1. General
3.2. Preparation of Graphene/Porphyrin Hybrid Materials
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Skachko, I.; Barker, A.; Andrei, E.Y. Approaching ballistic transport in suspended graphene. Nat. Nanotechnol. 2008, 3, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, Y.-W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum hall effect and berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhang, L.; Zhou, C. Review of chemical vapor deposition of graphene and related applications. Acc. Chem. Res. 2013, 46, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hernández, Y.; Feng, X.; Müllen, K. From nanographene and graphene nanoribbons to graphene sheets: Chemical synthesis. Angew. Chem. Int. Ed. 2012, 51, 7640–7654. [Google Scholar] [CrossRef]
- Eigler, S.; Hirsch, A. Chemistry with graphene and graphene oxide—Challenges for synthetic chemists. Angew. Chem. Int. Ed. 2014, 53, 7720–7738. [Google Scholar] [CrossRef]
- Pénicaud, A.; Drummond, C. Deconstructing graphite: Graphenide solutions. Acc. Chem. Res. 2012, 46, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, E.; Giacalone, F.; Prato, M. Non-conventional methods and media for the activation and manipulation of carbon nanoforms. Chem. Soc. Rev. 2014, 43, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Quintana, M.; Vázquez, E.; Prato, M. Organic functionalization of graphene in dispersions. Acc. Chem. Res. 2013, 46, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hernández, Y.; Schlierf, A.; Felten, A.; Eckmann, A.; Johal, S.; Louette, P.; Pireaux, J.J.; Feng, X.; Müllen, K.; et al. A simple method for graphene production based on exfoliation of graphite in water using 1-pyrenesulfonic acid sodium salt. Carbon 2013, 53, 357–365. [Google Scholar] [CrossRef]
- Coleman, J.N. Liquid exfoliation of defect-free graphene. Acc. Chem. Res. 2012, 46, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Sreeprasad, T.S.; Berry, V. How do the electrical properties of graphene change with its functionalization? Small 2013, 9, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Roppolo, I.; Chiappone, A.; Bejtka, K.; Celasco, E.; Chiodoni, A.; Giorgis, F.; Sangermano, M.; Porro, S. A powerful tool for graphene functionalization: Benzophenone mediated UV-grafting. Carbon 2014, 77, 226–235. [Google Scholar] [CrossRef]
- Zhang, M.; Parajuli, R.R.; Mastrogiovanni, D.; Dai, B.; Lo, P.; Cheung, W.; Brukh, R.; Chiu, P.L.; Zhou, T.; Liu, Z.; et al. Production of graphene sheets by direct dispersion with aromatic healing agents. Small 2010, 6, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, X.; Boulos, R.A.; Md Yasin, F.; Lu, H.; Raston, C.; Zhang, H. Pyrene-conjugated hyaluronan facilitated exfoliation and stabilisation of low dimensional nanomaterials in water. Chem. Commun. 2013, 49, 4845–4847. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Wang, Y.-B. Noncovalent π···π interaction between graphene and aromatic molecule: Structure, energy, and nature. J. Chem. Phys. 2014. [Google Scholar] [CrossRef]
- Ghosh, A.; Rao, K.V.; George, S.J.; Rao, C.N.R. Noncovalent functionalization, exfoliation, and solubilization of graphene in water by employing a fluorescent coronene carboxylate. Chem. Eur. J. 2010, 16, 2700–2704. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.D.; Malig, J.; Brenner, W.; Jux, N.; Guldi, D.M. Electron accepting porphycenes on graphene. Adv. Mater. 2013, 25, 2600–2605. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Liu, S.; Zhi, L. Chemical approaches toward graphene-based nanomaterials and their applications in energy-related areas. Small 2012, 8, 630–646. [Google Scholar] [CrossRef] [PubMed]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Díaz, M.V.; de la Torre, G.; Torres, T. Lighting porphyrins and phthalocyanines for molecular photovoltaics. Chem. Commun. 2010, 46, 7090–7108. [Google Scholar] [CrossRef]
- Benniston, A.C. Porphyrin linked poly(pyridyl)-based conjugates as artificial photosynthetic reaction centre models. Phys. Chem. Chem. Phys. 2007, 9, 5739–5747. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Du, F.; Ren, D.; Chen, Y.; Zheng, J.; Liu, Z.; Tian, J. Covalently porphyrin-functionalized single-walled carbon nanotubes: A novel photoactive and optical limiting donor-acceptor nanohybrid. J. Mater. Chem. 2006, 16, 3021–3030. [Google Scholar] [CrossRef]
- Xu, H.; Wu, P.; Liao, C.; Lv, C.; Gu, Z. Controlling the morphology and optoelectronic properties of graphene hybrid materials by porphyrin interactions. Chem. Commun. 2014, 50, 8951–8954. [Google Scholar] [CrossRef]
- Aly, S.M.; Parida, M.R.; Alarousu, E.; Mohammed, O.F. Ultrafast electron injection at the cationic porphyrin-graphene interface assisted by molecular flattening. Chem. Commun. 2014, 50, 10452–10455. [Google Scholar] [CrossRef]
- Malig, J.; Stephenson, A.W.I.; Wagner, P.; Wallace, G.G.; Officer, D.L.; Guldi, D.M. Direct exfoliation of graphite with a porphyrin—Creating functionalizable nanographene hybrids. Chem. Commun. 2012, 48, 8745–8747. [Google Scholar] [CrossRef]
- Kiessling, D.; Costa, R.D.; Katsukis, G.; Malig, J.; Lodermeyer, F.; Feihl, S.; Roth, A.; Wibmer, L.; Kehrer, M.; Volland, M.; et al. Novel nanographene/porphyrin hybrids—Preparation, characterization, and application in solar energy conversion schemes. Chem. Sci. 2013, 4, 3085–3098. [Google Scholar] [CrossRef]
- Geng, J.; Kong, B.-S.; Yang, S.B.; Jung, H.-T. Preparation of graphene relying on porphyrin exfoliation of graphite. Chem. Commun. 2010, 46, 5091–5093. [Google Scholar] [CrossRef]
- Hernández, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, F.G.; Isla, H.; Aragó, J.; Ortí, E.; Pérez, E.M.; Martín, N. Exploiting multivalent nanoparticles for the supramolecular functionalization of graphene with a nonplanar recognition motif. Chem. Eur. J. 2013, 19, 9843–9848. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.; O’Neill, A.; Lotya, M.; De, S.; Coleman, J.N. High-concentration solvent exfoliation of graphene. Small 2010, 6, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Murray, R.W. Quenching of [Ru(bpy)3]2+ fluorescence by binding to au nanoparticles. Langmuir 2002, 18, 7077–7081. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Z.; Xiao, B.; Lu, Y.; Du, Y.; Yang, P.; Wang, X. Surfactant assistance in improvement of photocatalytic hydrogen production with the porphyrin noncovalently functionalized graphene nanocomposite. ACS Appl. Mater. Interfaces 2013, 5, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Ling, X.; Fang, Y.; Zhang, J.; Liu, Z. Graphene as a substrate to suppress fluorescence in resonance raman spectroscopy. J. Am. Chem. Soc. 2009, 131, 9890–9891. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.R.; Biswas, K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene, the new nanocarbon. J. Mater. Chem. 2009, 19, 2457–2469. [Google Scholar] [CrossRef]
- Das, B.; Voggu, R.; Rout, C.S.; Rao, C.N.R. Changes in the electronic structure and properties of graphene induced by molecular charge-transfer. Chem. Commun. 2008, 5155–5157. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal, M.M.; Pérez, E.M. One-Pot Exfoliation of Graphite and Synthesis of Nanographene/Dimesitylporphyrin Hybrids. Int. J. Mol. Sci. 2015, 16, 10704-10714. https://doi.org/10.3390/ijms160510704
Bernal MM, Pérez EM. One-Pot Exfoliation of Graphite and Synthesis of Nanographene/Dimesitylporphyrin Hybrids. International Journal of Molecular Sciences. 2015; 16(5):10704-10714. https://doi.org/10.3390/ijms160510704
Chicago/Turabian StyleBernal, M. Mar, and Emilio M. Pérez. 2015. "One-Pot Exfoliation of Graphite and Synthesis of Nanographene/Dimesitylporphyrin Hybrids" International Journal of Molecular Sciences 16, no. 5: 10704-10714. https://doi.org/10.3390/ijms160510704
APA StyleBernal, M. M., & Pérez, E. M. (2015). One-Pot Exfoliation of Graphite and Synthesis of Nanographene/Dimesitylporphyrin Hybrids. International Journal of Molecular Sciences, 16(5), 10704-10714. https://doi.org/10.3390/ijms160510704