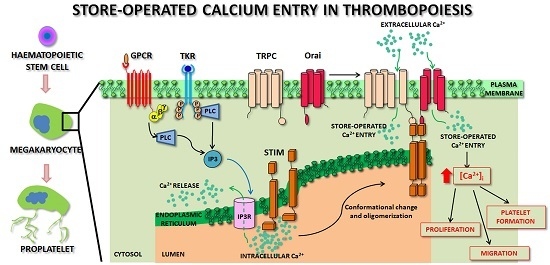
Pathophysiological Significance of Store-Operated Calcium Entry in Megakaryocyte Function: Opening New Paths for Understanding the Role of Calcium in Thrombopoiesis
Abstract
:1. Introduction
2. Dissecting the Molecular Mechanisms of Store-Operated Calcium Entry
2.1. Stromal Interaction Molecules: The Calcium Sensor of Intracellular Stores
2.2. The Interplay between Stromal Interaction Molecule and Orai Activates the Complex Choreography of Store-Operated Calcium Entry
3. Transient Receptor Potential Canonical Channels: Additional Components of Store-Operated Calcium Entry
4. Thrombopoiesis: The Long Route of Megakaryocytes to Platelet Production
5. Biogenesis of Store-Operated Calcium Entry during Thrombopoiesis: Biological Significance in Physiology and Pathology
5.1. Development of Endoplasmic Reticulum and Endoplasmic Reticulum-Related Proteins in Megakaryocytes
5.2. Expression and Function of Transient Receptor Potential Canonical during Megakaryocyte Differentiation
5.3. NF-κB Pathway Is a Major Regulator of Orai Expression in Megakaryocytes
5.4. Store-Operated Calcium Entry Finely Regulates Physiological Megakaryocyte Functions
5.5. Over-Activated Store-Operated Calcium Entry Is Observed in Pathological Thrombopoiesis
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| ATP | Adenosine triphosphate |
| Ca2+ | Calcium |
| [Ca2+]i | Cytoplasmic calcium concentration |
| CALR | Calreticulin |
| CC | Coiled-coil |
| CD | Cluster of differentiation |
| CDI | Calcium-dependent inactivation |
| cEF | Canonical EF-hand domain |
| CIRB | Calmodulin/inositol 1,4,5-trisphosphate receptor binding region |
| CRAC | Calcium-release activated calcium channel |
| Cs+ | Cesium |
| c-Mpl | Thrombopoietin receptor |
| DAG | Diacylglycerol |
| DMS | Demarcation membrane system |
| dOrai | Drosophila Orai |
| ECM | Extracellular matrix |
| Erev | Reversal potential |
| ET | Essential thrombocythemia |
| GPCR | G-protein coupled receptor |
| hEF | Hidden non-canonical EF-hand domain |
| HSC | Hematopoietic stem cells |
| HSG | Human salivary gland |
| ICRAC | Calcium release-activated calcium current |
| IP3 | Inositol 1,4,5-trisphosphate |
| IP3R | Inositol 1,4,5-trisphosphate receptor |
| ISOC | Store-operated activated calcium current |
| JAK2 | Janus kinase 2 |
| K+ | Potassium |
| MPN | Phildelphia-negative myeloproliferative neoplasm |
| Na+ | Sodium |
| NCX | Sodium/calcium exchanger |
| NF-κB | Nuclear factor κ-light-chain-enhancer of activated B cells |
| NMDAR | Ionotropic N-methyl-d-aspartate receptor |
| PDGF | Platelet derived growth factor |
| PF4 | Platelet factor 4 |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PKC | Protein kinase C |
| PKG | Protein kinase G |
| PLC | Phospholipase C |
| PMCA | Plasma membrane calcium-ATPase |
| PMF | Primary myelofibrosis |
| RNA | Ribonucleic acid |
| ROC | Receptor-operated channel |
| RyR | Ryanodine receptors |
| SAM | Sterile-α motif |
| SDF-1α | Stromal derived factor-1α |
| SERCA | Sarco/endoplasmic reticulum calcium-ATPase |
| SGK1 | Serum- and glucocorticoid-inducible kinase 1 |
| SMOC | Second messenger-operated channel |
| SOC | Store-operated channel |
| SOCE | Store-operated calcium entry |
| SR/ER | Sarco/endoplasmic reticulum |
| STIM | Stromal interaction molecule |
| TGF-β1 | Transforming growth factor-β1 |
| TKR | Tyrosine kinase receptor |
| TM | Transmembrane |
| TPO | Thrombopoietin |
| TRPA-1 | Transient receptor potential ankyrin-1 |
| TRPC | Transient receptor potential canonical |
| TRPV | Transient receptor potential vanilloid |
| VEGF | Vascular endothelial growth factor |
| VOC | Voltage-operated channel |
| vWF | Von Willebrand Factor |
References
- Carafoli, E. Calcium signaling: A tale for all seasons. Proc. Natl. Acad. Sci. USA 2002, 99, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozzan, T.; Rizzuto, R.; Volpe, P.; Meldolesi, J. Molecular and cellular physiology of intracellular calcium stores. Physiol. Rev. 1994, 74, 595–636. [Google Scholar] [PubMed]
- Sorrentino, V.; Rizzuto, R. Molecular genetics of Ca2+ stores and intracellular Ca2+ signalling. Trends Pharmacol. Sci. 2001, 22, 459–464. [Google Scholar] [CrossRef]
- Lim, D.; Bertoli, A.; Sorgato, M.C.; Moccia, F. Generation and usage of aequorin lentiviral vectors for Ca2+ measurement in sub-cellular compartments of hard-to-transfect cells. Cell Calcium 2016, 59, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 1995, 80, 259–268. [Google Scholar] [CrossRef]
- Moccia, F.; Nusco, G.A.; Lim, D.; Ercolano, E.; Gragnaniello, G.; Brown, E.R.; Santella, L. Ca2+ signalling and membrane current activated by cADPr in starfish oocytes. Pflugers Arch. 2003, 446, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Ronco, V.; Potenza, D.M.; Denti, F.; Vullo, S.; Gagliano, G.; Tognolina, M.; Guerra, G.; Pinton, P.; Genazzani, A.A.; Mapelli, L.; et al. A novel Ca2+-mediated cross-talk between endoplasmic reticulum and acidic organelles: Implications for NAADP-dependent Ca2+ signalling. Cell Calcium 2015, 57, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Prakriya, M.; Lewis, R.S. Store-operated calcium channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef] [PubMed]
- Hooper, R.; Rothberg, B.S.; Soboloff, J. Neuronal stimulation at rest. Sci. Signal. 2014, 7, pe18. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Zuccolo, E.; Soda, T.; Tanzi, F.; Guerra, G.; Mapelli, L.; Lodola, F.; D′Angelo, E. STIM and Orai proteins in neuronal Ca2+ signaling and excitability. Front. Cell. Neurosci. 2015, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Locke, E.G.; Bonilla, M.; Liang, L.; Takita, Y.; Cunningham, K.W. A homolog of voltage-gated Ca2+ channels stimulated by depletion of secretory Ca2+ in yeast. Mol. Cell. Biol. 2000, 20, 6686–6694. [Google Scholar] [CrossRef] [PubMed]
- Wes, P.D.; Chevesich, J.; Jeromin, A.; Rosenberg, C.; Stetten, G.; Montell, C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc. Natl. Acad. Sci. USA 1995, 92, 9652–9656. [Google Scholar] [CrossRef] [PubMed]
- Putney, J.W. A model for receptor-regulated calcium entry. Cell Calcium 1986, 7, 1–12. [Google Scholar] [CrossRef]
- Takemura, H.; Putney, J.W. Capacitative calcium entry in parotid acinar cells. Biochem. J. 1989, 258, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Feske, S. Immunodeficiency due to defects in store-operated calcium entry. Ann. N. Y. Acad. Sci. 2011, 1238, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.I.; Ong, H.L.; Liu, X.; Alevizos, I.; Ambudkar, I.S. Up-regulation of store-operated Ca2+ entry and nuclear factor of activated T cells promote the acinar phenotype of the primary human salivary gland cells. J. Biol. Chem. 2016, 291, 8709–8720. [Google Scholar] [CrossRef] [PubMed]
- Toth, A.B.; Shum, A.K.; Prakriya, M. Regulation of neurogenesis by calcium signaling. Cell Calcium 2016, 59, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Zuccolo, E.; Poletto, V.; Turin, I.; Guerra, G.; Pedrazzoli, P.; Rosti, V.; Porta, C.; Montagna, D. Targeting STIM and Orai proteins as an alternative approach in anticancer therapy. Curr. Med. Chem. 2016, 23, 3450–3480. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Dragoni, S.; Lodola, F.; Bonetti, E.; Bottino, C.; Guerra, G.; Laforenza, U.; Rosti, V.; Tanzi, F. Store-dependent Ca2+ entry in endothelial progenitor cells as a perspective tool to enhance cell-based therapy and adverse tumour vascularization. Curr. Med. Chem. 2012, 19, 5802–5818. [Google Scholar] [CrossRef] [PubMed]
- Stiber, J.; Hawkins, A.; Zhang, Z.S.; Wang, S.; Burch, J.; Graham, V.; Ward, C.C.; Seth, M.; Finch, E.; Malouf, N.; et al. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat. Cell Biol. 2008, 10, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Berra-Romani, R.; Mazzocco-Spezzia, A.; Pulina, M.V.; Golovina, V.A. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am. J. Physiol. Cell Physiol. 2008, 295, C779–C790. [Google Scholar] [CrossRef] [PubMed]
- Potier, M.; Gonzalez, J.C.; Motiani, R.K.; Abdullaev, I.F.; Bisaillon, J.M.; Singer, H.A.; Trebak, M. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: Role in proliferation and migration. FASEB J. 2009, 23, 2425–2437. [Google Scholar] [CrossRef] [PubMed]
- Collins, H.E.; Zhu-Mauldin, X.; Marchase, R.B.; Chatham, J.C. STIM1/Orai1-mediated SOCE: Current perspectives and potential roles in cardiac function and pathology. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H446–H458. [Google Scholar] [CrossRef] [PubMed]
- Courjaret, R.; Machaca, K. STIM and Orai in cellular proliferation and division. Front. Biosci. 2012, 4, 331–341. [Google Scholar] [CrossRef]
- Kar, P.; Parekh, A. STIM proteins, Orai1 and gene expression. Channels 2013, 7, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Di Buduo, C.A.; Moccia, F.; Battiston, M.; de Marco, L.; Mazzucato, M.; Moratti, R.; Tanzi, F.; Balduini, A. The importance of calcium in the regulation of megakaryocyte function. Haematologica 2014, 99, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Parekh, A.B.; Putney, J.W. Store-operated calcium channels. Physiol. Rev. 2005, 85, 757–810. [Google Scholar] [CrossRef] [PubMed]
- Hoth, M.; Penner, R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 1992, 355, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Penner, R.; Fasolato, C.; Hoth, M. Calcium influx and its control by calcium release. Curr. Opin. Neurobiol. 1993, 3, 368–374. [Google Scholar] [CrossRef]
- Liou, J.; Kim, M.L.; Heo, W.D.; Jones, J.T.; Myers, J.W.; Ferrell, J.E.; Meyer, T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005, 15, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Roos, J.; DiGregorio, P.J.; Yeromin, A.V.; Ohlsen, K.; Lioudyno, M.; Zhang, S.; Safrina, O.; Kozak, J.A.; Wagner, S.L.; Cahalan, M.D.; et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005, 169, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Feske, S.; Gwack, Y.; Prakriya, M.; Srikanth, S.; Puppel, S.H.; Tanasa, B.; Hogan, P.G.; Lewis, R.S.; Daly, M.; Rao, A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006, 441, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Yeromin, A.V.; Zhang, X.H.; Yu, Y.; Safrina, O.; Penna, A.; Roos, J.; Stauderman, K.A.; Cahalan, M.D. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc. Natl. Acad. Sci. USA 2006, 103, 9357–9362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Yu, Y.; Roos, J.; Kozak, J.A.; Deerinck, T.J.; Ellisman, M.H.; Stauderman, K.A.; Cahalan, M.D. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 2005, 437, 902–905. [Google Scholar] [CrossRef] [PubMed]
- Spassova, M.A.; Soboloff, J.; He, L.P.; Xu, W.; Dziadek, M.A.; Gill, D.L. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc. Natl. Acad. Sci. USA 2006, 103, 4040–4045. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.C.; Dehaven, W.I.; Smyth, J.T.; Wedel, B.; Boyles, R.R.; Bird, G.S.; Putney, J.W. Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, STIM1. J. Biol. Chem. 2006, 281, 24979–24990. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.M.; Buchanan, J.; Luik, R.M.; Lewis, R.S. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol. 2006, 174, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Brandman, O.; Liou, J.; Park, W.S.; Meyer, T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 2007, 131, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Manji, S.S.; Parker, N.J.; Williams, R.T.; van Stekelenburg, L.; Pearson, R.B.; Dziadek, M.; Smith, P.J. STIM1: A novel phosphoprotein located at the cell surface. Biochim. Biophys. Acta 2000, 1481, 147–155. [Google Scholar] [CrossRef]
- Shim, A.H.; Tirado-Lee, L.; Prakriya, M. Structural and functional mechanisms of CRAC channel regulation. J. Mol. Biol. 2015, 427, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Stathopulos, P.B.; Li, G.Y.; Plevin, M.J.; Ames, J.B.; Ikura, M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J. Biol. Chem. 2006, 281, 35855–35862. [Google Scholar] [CrossRef] [PubMed]
- Soboloff, J.; Spassova, M.A.; Hewavitharana, T.; He, L.P.; Xu, W.; Johnstone, L.S.; Dziadek, M.A.; Gill, D.L. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ entry. Curr. Biol. 2006, 16, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.T.; Manji, S.S.; Parker, N.J.; Hancock, M.S.; van Stekelenburg, L.; Eid, J.P.; Senior, P.V.; Kazenwadel, J.S.; Shandala, T.; Saint, R.; et al. Identification and characterization of the STIM (stromal interaction molecule) gene family: Coding for a novel class of transmembrane proteins. Biochem. J. 2001, 357, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Stathopulos, P.B.; Li, G.Y.; Ikura, M. Biophysical characterization of the EF-hand and SAM domain containing Ca2+ sensory region of STIM1 and STIM2. Biochem. Biophys. Res. Commun. 2008, 369, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Darbellay, B.; Arnaudeau, S.; Bader, C.R.; Konig, S.; Bernheim, L. STIM1l is a new actin-binding splice variant involved in fast repetitive Ca2+ release. J. Cell Biol. 2011, 194, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Miederer, A.M.; Alansary, D.; Schwär, G.; Lee, P.H.; Jung, M.; Helms, V.; Niemeyer, B.A. A STIM2 splice variant negatively regulates store-operated calcium entry. Nat. Commun. 2015, 6, 6899. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Yen, M.; Sadaghiani, A.M.; Malmersjö, S.; Park, C.Y.; Dolmetsch, R.E.; Lewis, R.S. Alternative splicing converts STIM2 from an activator to an inhibitor of store-operated calcium channels. J. Cell Biol. 2015, 209, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Hoth, M.; Niemeyer, B.A. The neglected CRAC proteins: Orai2, Orai3, and STIM2. Curr. Top. Membr. 2013, 71, 237–271. [Google Scholar] [PubMed]
- Frischauf, I.; Schindl, R.; Bergsmann, J.; Derler, I.; Fahrner, M.; Muik, M.; Fritsch, R.; Lackner, B.; Groschner, K.; Romanin, C. Cooperativeness of Orai cytosolic domains tunes subtype-specific gating. J. Biol. Chem. 2011, 286, 8577–8584. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Murakami, M.; Watanabe, H.; Hasegawa, H.; Ohba, T.; Munehisa, Y.; Nobori, K.; Ono, K.; Iijima, T.; Ito, H. Essential role of the N-terminus of murine Orai1 in store-operated Ca2+ entry. Biochem. Biophys. Res. Commun. 2007, 356, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Cahalan, M.D.; Zhang, S.L.; Yeromin, A.V.; Ohlsen, K.; Roos, J.; Stauderman, K.A. Molecular basis of the CRAC channel. Cell Calcium 2007, 42, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Frischauf, I.; Muik, M.; Derler, I.; Bergsmann, J.; Fahrner, M.; Schindl, R.; Groschner, K.; Romanin, C. Molecular determinants of the coupling between STIM1 and Orai channels: Differential activation of Orai1–3 channels by a STIM1 coiled-coil mutant. J. Biol. Chem. 2009, 284, 21696–21706. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Pedi, L.; Diver, M.M.; Long, S.B. Crystal structure of the calcium release-activated calcium channel Orai. Science 2012, 338, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Derler, I.; Plenk, P.; Fahrner, M.; Muik, M.; Jardin, I.; Schindl, R.; Gruber, H.J.; Groschner, K.; Romanin, C. The extended transmembrane Orai1 N-terminal (ETON) region combines binding interface and gate for Orai1 activation by STIM1. J. Biol. Chem. 2013, 288, 29025–29034. [Google Scholar] [CrossRef] [PubMed]
- McNally, B.A.; Prakriya, M. Permeation, selectivity and gating in store-operated CRAC channels. J. Physiol. 2012, 590, 4179–4191. [Google Scholar] [CrossRef] [PubMed]
- Gudlur, A.; Quintana, A.; Zhou, Y.; Hirve, N.; Mahapatra, S.; Hogan, P.G. STIM1 triggers a gating rearrangement at the extracellular mouth of the Orai1 channel. Nat. Commun. 2014, 5, 5164. [Google Scholar] [CrossRef] [PubMed]
- Derler, I.; Jardin, I.; Romanin, C. Molecular mechanisms of STIM/Orai communication. Am. J. Physiol. Cell Physiol. 2016, 310, C643–C662. [Google Scholar] [PubMed]
- Parekh, A.B. Store-operated CRAC channels: Function in health and disease. Nat. Rev. Drug Discov. 2010, 9, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Feske, S. Orai1 and STIM1 deficiency in human and mice: Roles of store-operated Ca2+ entry in the immune system and beyond. Immunol. Rev. 2009, 231, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Abdullaev, I.F.; Bisaillon, J.M.; Potier, M.; Gonzalez, J.C.; Motiani, R.K.; Trebak, M. STIM1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ. Res. 2008, 103, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Halligan, K.E.; Zhang, X.; Bisaillon, J.M.; Gonzalez-Cobos, J.C.; Motiani, R.K.; Hu, G.; Vincent, P.A.; Zhou, J.; Barroso, M.; et al. Orai1-mediated ICRAC is essential for neointima formation after vascular injury. Circ. Res. 2011, 109, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Stanisz, H.; Stark, A.; Kilch, T.; Schwarz, E.C.; Müller, C.S.; Peinelt, C.; Hoth, M.; Niemeyer, B.A.; Vogt, T.; Bogeski, I. Orai1 Ca2+ channels control endothelin-1-induced mitogenesis and melanogenesis in primary human melanocytes. J. Investig. Dermatol. 2012, 132, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Ohana, L.; Newell, E.W.; Stanley, E.F.; Schlichter, L.C. The Ca2+ release-activated Ca2+ current ICRAC mediates store-operated Ca2+ entry in rat microglia. Channels 2009, 3, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Barritt, G.J.; Litjens, T.L.; Castro, J.; Aromataris, E.; Rychkov, G.Y. Store-operated Ca2+ channels and microdomains of Ca2+ in liver cells. Clin. Exp. Pharmacol. Physiol. 2009, 36, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Stathopulos, P.B.; Zheng, L.; Ikura, M. Stromal interaction molecule (STIM) 1 and STIM2 calcium sensing regions exhibit distinct unfolding and oligomerization kinetics. J. Biol. Chem. 2009, 284, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Bakowski, D.; di Capite, J.; Nelson, C.; Parekh, A.B. Different agonists recruit different stromal interaction molecule proteins to support cytoplasmic Ca2+ oscillations and gene expression. Proc. Natl. Acad. Sci. USA 2012, 109, 6969–6974. [Google Scholar] [CrossRef] [PubMed]
- Hoover, P.J.; Lewis, R.S. Stoichiometric requirements for trapping and gating of Ca2+ release-activated Ca2+ (CRAC) channels by stromal interaction molecule 1 (STIM1). Proc. Natl. Acad. Sci. USA 2011, 108, 13299–13304. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, L.; Deng, Y.; Ji, W.; Du, W.; Xu, P.; Chen, L.; Xu, T. Graded activation of CRAC channel by binding of different numbers of STIM1 to Orai1 subunits. Cell Res. 2011, 21, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Stathopulos, P.B.; Schindl, R.; Fahrner, M.; Zheng, L.; Gasmi-Seabrook, G.M.; Muik, M.; Romanin, C.; Ikura, M. STIM1/Orai1 coiled-coil interplay in the regulation of store-operated calcium entry. Nat. Commun. 2013, 4, 2963. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Montell, C. TRP channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Birnbaumer, L. The TRPC class of ion channels: A critical review of their roles in slow, sustained increases in intracellular Ca2+ concentrations. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 395–426. [Google Scholar] [CrossRef] [PubMed]
- Lockwich, T.P.; Liu, X.; Singh, B.B.; Jadlowiec, J.; Weiland, S.; Ambudkar, I.S. Assembly of TRP1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J. Biol. Chem. 2000, 275, 11934–11942. [Google Scholar] [CrossRef] [PubMed]
- Lockwich, T.; Singh, B.B.; Liu, X.; Ambudkar, I.S. Stabilization of cortical actin induces internalization of transient receptor potential 3 (TRP3)-associated caveolar Ca2+ signaling complex and loss of Ca2+ influx without disruption of TRP3-inositol trisphosphate receptor association. J. Biol. Chem. 2001, 276, 42401–42408. [Google Scholar] [CrossRef] [PubMed]
- Vannier, B.; Zhu, X.; Brown, D.; Birnbaumer, L. The membrane topology of human transient receptor potential 3 as inferred from glycosylation-scanning mutagenesis and epitope immunocytochemistry. J. Biol. Chem. 1998, 273, 8675–8679. [Google Scholar] [CrossRef] [PubMed]
- Strübing, C.; Krapivinsky, G.; Krapivinsky, L.; Clapham, D.E. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J. Biol. Chem. 2003, 278, 39014–39019. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Schaefer, M.; Schultz, G.; Gudermann, T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. USA 2002, 99, 7461–7466. [Google Scholar] [CrossRef] [PubMed]
- Wedel, B.J.; Vazquez, G.; McKay, R.R.; Bird, G.S.J.; Putney, J.W. A calmodulin/inositol 1,4,5-trisphosphate (IP3) receptor-binding region targets TRPC3 to the plasma membrane in a calmodulin/IP3 receptor-independent process. J. Biol. Chem. 2003, 278, 25758–25765. [Google Scholar] [CrossRef] [PubMed]
- Engelke, M.; Friedrich, O.; Budde, P.; Schäfer, C.; Niemann, U.; Zitt, C.; Jüngling, E.; Rocks, O.; Lückhoff, A.; Frey, J. Structural domains required for channel function of the mouse transient receptor potential protein homologue TRP1β. FEBS Lett. 2002, 523, 193–199. [Google Scholar] [CrossRef]
- Singh, B.B.; Liu, X.; Tang, J.; Zhu, M.X.; Ambudkar, I.S. Calmodulin regulates Ca2+-dependent feedback inhibition of store-operated Ca2+ influx by interaction with a site in the C terminus of TRPC1. Mol. Cell 2002, 9, 739–750. [Google Scholar] [CrossRef]
- Brazer, S.C.; Singh, B.B.; Liu, X.; Swaim, W.; Ambudkar, I.S. Caveolin-1 contributes to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1. J. Biol. Chem. 2003, 278, 27208–27215. [Google Scholar] [CrossRef] [PubMed]
- Dohke, Y.; Oh, Y.S.; Ambudkar, I.S.; Turner, R.J. Biogenesis and topology of the transient receptor potential Ca2+ channel TRPC1. J. Biol. Chem. 2004, 279, 12242–12248. [Google Scholar] [CrossRef] [PubMed]
- Kwan, H.Y.; Huang, Y.; Yao, X. Regulation of canonical transient receptor potential isoform 3 (TRPC3) channel by protein kinase G. Proc. Natl. Acad. Sci. USA 2004, 101, 2625–2630. [Google Scholar] [CrossRef] [PubMed]
- Trebak, M.; Hempel, N.; Wedel, B.J.; Smyth, J.T.; Bird, G.S.; Putney, J.W. Negative regulation of TRPC3 channels by protein kinase C-mediated phosphorylation of serine 712. Mol. Pharmacol. 2005, 67, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Sinkins, W.G.; Schilling, W.P. Selective association of TRPC channel subunits in rat brain synaptosomes. J. Biol. Chem. 2002, 277, 48303–48310. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Cheng, K.T.; Wong, C.O.; O′Neil, R.G.; Birnbaumer, L.; Ambudkar, I.S.; Yao, X. Heteromeric TRPV4-c1 channels contribute to store-operated Ca2+ entry in vascular endothelial cells. Cell Calcium 2011, 50, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Schindl, R.; Fritsch, R.; Jardin, I.; Frischauf, I.; Kahr, H.; Muik, M.; Riedl, M.C.; Groschner, K.; Romanin, C. Canonical transient receptor potential (TRPC) 1 acts as a negative regulator for vanilloid TRPV6-mediated Ca2+ influx. J. Biol. Chem. 2012, 287, 35612–35620. [Google Scholar] [CrossRef] [PubMed]
- Albarrán, L.; Lopez, J.J.; Dionisio, N.; Smani, T.; Salido, G.M.; Rosado, J.A. Transient receptor potential ankyrin-1 (TRPA1) modulates store-operated Ca2+ entry by regulation of STIM1-Orai1 association. Biochim. Biophys. Acta 2013, 1833, 3025–3034. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.T.; Ong, H.L.; Liu, X.; Ambudkar, I.S. Contribution and regulation of TRPC channels in store-operated Ca2+ entry. Curr. Top. Membr. 2013, 71, 149–179. [Google Scholar] [PubMed]
- Ong, H.L.; de Souza, L.B.; Ambudkar, I.S. Role of TRPC channels in store-operated calcium entry. Adv. Exp. Med. Biol. 2016, 898, 87–109. [Google Scholar] [PubMed]
- Liu, X.; Singh, B.B.; Ambudkar, I.S. TRPC1 is required for functional store-operated Ca2+ channels. Role of acidic amino acid residues in the S5–S6 region. J. Biol. Chem. 2003, 278, 11337–11343. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, K.T.; Bandyopadhyay, B.C.; Pani, B.; Dietrich, A.; Paria, B.C.; Swaim, W.D.; Beech, D.; Yildrim, E.; Singh, B.B.; et al. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1−/− mice. Proc. Natl. Acad. Sci. USA 2007, 104, 17542–17547. [Google Scholar] [CrossRef] [PubMed]
- Lodola, F.; Laforenza, U.; Bonetti, E.; Lim, D.; Dragoni, S.; Bottino, C.; Ong, H.L.; Guerra, G.; Ganini, C.; Massa, M.; et al. Store-operated Ca2+ entry is remodelled and controls in vitro angiogenesis in endothelial progenitor cells isolated from tumoral patients. PLoS ONE 2012, 7, e42541. [Google Scholar] [CrossRef] [PubMed]
- Fatherazi, S.; Presland, R.B.; Belton, C.M.; Goodwin, P.; Al-Qutub, M.; Trbic, Z.; Macdonald, G.; Schubert, M.M.; Izutsu, K.T. Evidence that TRPC4 supports the calcium selective ICRAC-like current in human gingival keratinocytes. Pflugers Arch. 2007, 453, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Freichel, M.; Suh, S.H.; Pfeifer, A.; Schweig, U.; Trost, C.; Weissgerber, P.; Biel, M.; Philipp, S.; Freise, D.; Droogmans, G.; et al. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat. Cell Biol. 2001, 3, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Mergler, S.; Sun, X.; Wang, Z.; Lu, L.; Bonanno, J.A.; Pleyer, U.; Reinach, P.S. TRPC4 knockdown suppresses epidermal growth factor-induced store-operated channel activation and growth in human corneal epithelial cells. J. Biol. Chem. 2005, 280, 32230–32237. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, G.; Wedel, B.J.; Trebak, M.; St John Bird, G.; Putney, J.W. Expression level of the canonical transient receptor potential 3 (TRPC3) channel determines its mechanism of activation. J. Biol. Chem. 2003, 278, 21649–21654. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.L.; de Souza, L.B.; Cheng, K.T.; Ambudkar, I.S. Physiological functions and regulation of TRPC channels. Handb. Exp. Pharmacol. 2014, 223, 1005–1034. [Google Scholar] [PubMed]
- Zeng, W.; Yuan, J.P.; Kim, M.S.; Choi, Y.J.; Huang, G.N.; Worley, P.F.; Muallem, S. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol. Cell 2008, 32, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Sundivakkam, P.C.; Freichel, M.; Singh, V.; Yuan, J.P.; Vogel, S.M.; Flockerzi, V.; Malik, A.B.; Tiruppathi, C. The Ca2+ sensor stromal interaction molecule 1 (STIM1) is necessary and sufficient for the store-operated Ca2+ entry function of transient receptor potential canonical (TRPC) 1 and 4 channels in endothelial cells. Mol. Pharmacol. 2012, 81, 510–526. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.L.; Cheng, K.T.; Liu, X.; Bandyopadhyay, B.C.; Paria, B.C.; Soboloff, J.; Pani, B.; Gwack, Y.; Srikanth, S.; Singh, B.B.; et al. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J. Biol. Chem. 2007, 282, 9105–9116. [Google Scholar] [CrossRef] [PubMed]
- Jardin, I.; Lopez, J.J.; Salido, G.M.; Rosado, J.A. Orai1 mediates the interaction between STIM1 and hTRPC1 and regulates the mode of activation of hTRPC1-forming Ca2+ channels. J. Biol. Chem. 2008, 283, 25296–25304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Pan, L.J.; Zhang, Z.M. Functional interactions among STIM1, Orai1 and TRPC1 on the activation of socs in HL-7702 cells. Amino Acids 2010, 39, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.C.; McCormack, M.D.; Airey, J.A.; Singer, C.A.; Keller, P.S.; Shen, X.M.; Hume, J.R. TRPC1 and STIM1 mediate capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells. J. Physiol. 2009, 587, 2429–2442. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Bränström, R.; Berglund, E.; Höög, A.; Björklund, P.; Westin, G.; Larsson, C.; Farnebo, L.O.; Forsberg, L. Expression and association of TRPC subtypes with Orai1 and STIM1 in human parathyroid. J. Mol. Endocrinol. 2010, 44, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Almirza, W.H.; Peters, P.H.; van Zoelen, E.J.; Theuvenet, A.P. Role of TRPC channels, STIM1 and Orai1 in PGF2α-induced calcium signaling in NRK fibroblasts. Cell Calcium 2012, 51, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Berra-Romani, R.; Tanzi, F. Update on vascular endothelial Ca2+ signalling: A tale of ion channels, pumps and transporters. World J. Biol. Chem. 2012, 3, 127–158. [Google Scholar] [CrossRef] [PubMed]
- Salido, G.M.; Sage, S.O.; Rosado, J.A. TRPC channels and store-operated Ca2+ entry. Biochim. Biophys. Acta 2009, 1793, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.T.; Liu, X.; Ong, H.L.; Swaim, W.; Ambudkar, I.S. Local Ca2+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca2+ signals required for specific cell functions. PLoS Biol. 2011, 9, e1001025. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Kaushansky, K. Thrombopoiesis. Semin. Hematol. 2015, 52, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Woolthuis, C.M.; Park, C.Y. Hematopoietic stem/progenitor cell commitment to the megakaryocyte lineage. Blood 2016, 127, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Geddis, A.E.; Kaushansky, K. Immunology. The root of platelet production. Science 2007, 317, 1689–1691. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, I.S.; Kaushansky, K. Thrombopoietin from beginning to end. Br. J. Haematol. 2014, 165, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Italiano, J.E.; Lecine, P.; Shivdasani, R.A.; Hartwig, J.H. Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J. Cell Biol. 1999, 147, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Italiano, J.E.; Shivdasani, R.A. Megakaryocytes and beyond: The birth of platelets. J. Thromb. Haemost. 2003, 1, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Youssefian, T.; Cramer, E.M. Megakaryocyte dense granule components are sorted in multivesicular bodies. Blood 2000, 95, 4004–4007. [Google Scholar] [PubMed]
- Blair, P.; Flaumenhaft, R. Platelet α-granules: Basic biology and clinical correlates. Blood. Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Fava, R.A.; Casey, T.T.; Wilcox, J.; Pelton, R.W.; Moses, H.L.; Nanney, L.B. Synthesis of transforming growth factor-β 1 by megakaryocytes and its localization to megakaryocyte and platelet α-granules. Blood 1990, 76, 1946–1955. [Google Scholar] [PubMed]
- Hamada, T.; Möhle, R.; Hesselgesser, J.; Hoxie, J.; Nachman, R.L.; Moore, M.A.; Rafii, S. Transendothelial migration of megakaryocytes in response to stromal cell-derived factor 1 (SDF-1) enhances platelet formation. J. Exp. Med. 1998, 188, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Avecilla, S.T.; Hattori, K.; Heissig, B.; Tejada, R.; Liao, F.; Shido, K.; Jin, D.K.; Dias, S.; Zhang, F.; Hartman, T.E.; et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat. Med. 2004, 10, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Junt, T.; Schulze, H.; Chen, Z.; Massberg, S.; Goerge, T.; Krueger, A.; Wagner, D.D.; Graf, T.; Italiano, J.E.; Shivdasani, R.A.; et al. Dynamic visualization of thrombopoiesis within bone marrow. Science 2007, 317, 1767–1770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Orban, M.; Lorenz, M.; Barocke, V.; Braun, D.; Urtz, N.; Schulz, C.; von Brühl, M.L.; Tirniceriu, A.; Gaertner, F.; et al. A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis. J. Exp. Med. 2012, 209, 2165–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Buduo, C.A.; Wray, L.S.; Tozzi, L.; Malara, A.; Chen, Y.; Ghezzi, C.E.; Smoot, D.; Sfara, C.; Antonelli, A.; Spedden, E.; et al. Programmable 3D silk bone marrow niche for platelet generation ex vivo and modeling of megakaryopoiesis pathologies. Blood 2015, 125, 2254–2264. [Google Scholar] [CrossRef] [PubMed]
- Balduini, A.; di Buduo, C.A.; Kaplan, D.L. Translational approaches to functional platelet production ex vivo. Thromb. Haemost. 2016, 115, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Sim, X.; Poncz, M.; Gadue, P.; French, D.L. Understanding platelet generation from megakaryocytes: Implications for in vitro-derived platelets. Blood 2016, 127, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Di Buduo, C.A.; Currao, M.; Pecci, A.; Kaplan, D.L.; Balduini, C.L.; Balduini, A. Revealing eltrombopag’s promotion of human megakaryopoiesis through AKT/ERK-dependent pathway activation. Haematologica 2016, 125, 2254–2264. [Google Scholar]
- Long, M.W. Megakaryocyte differentiation events. Semin. Hematol. 1998, 35, 192–199. [Google Scholar] [PubMed]
- Nishimura, S.; Nagasaki, M.; Kunishima, S.; Sawaguchi, A.; Sakata, A.; Sakaguchi, H.; Ohmori, T.; Manabe, I.; Italiano, J.E.; Ryu, T.; et al. Il-1α induces thrombopoiesis through megakaryocyte rupture in response to acute platelet needs. J. Cell Biol. 2015, 209, 453–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Celso, C.; Fleming, H.E.; Wu, J.W.; Zhao, C.X.; Miake-Lye, S.; Fujisaki, J.; Côté, D.; Rowe, D.W.; Lin, C.P.; Scadden, D.T. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature 2009, 457, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Arai, F.; Hirao, A.; Ohmura, M.; Sato, H.; Matsuoka, S.; Takubo, K.; Ito, K.; Koh, G.Y.; Suda, T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 2004, 118, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Fliedner, T.M.; Calvo, W.; Klinnert, V.; Nothdurft, W.; Prümmer, O.; Raghavachar, A. Bone marrow structure and its possible significance for hematopoietic cell renewal. Ann. N. Y. Acad. Sci. 1985, 459, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Malara, A.; Gruppi, C.; Rebuzzini, P.; Visai, L.; Perotti, C.; Moratti, R.; Balduini, C.; Tira, M.E.; Balduini, A. Megakaryocyte-matrix interaction within bone marrow: New roles for fibronectin and factor XIII-A. Blood 2011, 117, 2476–2483. [Google Scholar] [CrossRef] [PubMed]
- Abbonante, V.; Gruppi, C.; Rubel, D.; Gross, O.; Moratti, R.; Balduini, A. Discoidin domain receptor 1 protein is a novel modulator of megakaryocyte-collagen interactions. J. Biol. Chem. 2013, 288, 16738–16746. [Google Scholar] [CrossRef] [PubMed]
- Balduini, A.; Pallotta, I.; Malara, A.; Lova, P.; Pecci, A.; Viarengo, G.; Balduini, C.L.; Torti, M. Adhesive receptors, extracellular proteins and myosin IIA orchestrate proplatelet formation by human megakaryocytes. J. Thromb. Haemost. 2008, 6, 1900–1907. [Google Scholar] [CrossRef] [PubMed]
- Malara, A.; Currao, M.; Gruppi, C.; Celesti, G.; Viarengo, G.; Buracchi, C.; Laghi, L.; Kaplan, D.L.; Balduini, A. Megakaryocytes contribute to the bone marrow-matrix environment by expressing fibronectin, type IV collagen, and laminin. Stem Cells 2014, 32, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Abbonante, V.; Gruppi, C.; Catarsi, P.; Avanzini, M.A.; Tira, M.E.; Barosi, G.; Rosti, V.; Balduini, A. Altered fibronectin expression and deposition by myeloproliferative neoplasm-derived mesenchymal stromal cells. Br. J. Haematol. 2015, 172, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Kuter, D.J.; Bain, B.; Mufti, G.; Bagg, A.; Hasserjian, R.P. Bone marrow fibrosis: Pathophysiology and clinical significance of increased bone marrow stromal fibres. Br. J. Haematol. 2007, 139, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Katoh, O.; Hyodo, H.; Kuramoto, A. Transforming growth factor-β regulates growth as well as collagen and fibronectin synthesis of human marrow fibroblasts. Br. J. Haematol. 1989, 72, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Abbonante, V.; di Buduo, C.A.; Gruppi, C.; Malara, A.; Gianelli, U.; Celesti, G.; Anselmo, A.; Laghi, L.; Vercellino, M.; Visai, L.; et al. Thrombopoietin/TGF-β1 loop regulates megakaryocyte extracellular matrix component synthesis. Stem Cells 2016, 34, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Badalucco, S.; di Buduo, C.A.; Campanelli, R.; Pallotta, I.; Catarsi, P.; Rosti, V.; Kaplan, D.L.; Barosi, G.; Massa, M.; Balduini, A. Involvement of TGFβ1 in autocrine regulation of proplatelet formation in healthy subjects and patients with primary myelofibrosis. Haematologica 2013, 98, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Balduini, A.; di Buduo, C.A.; Malara, A.; Lecchi, A.; Rebuzzini, P.; Currao, M.; Pallotta, I.; Jakubowski, J.A.; Cattaneo, M. Constitutively released adenosine diphosphate regulates proplatelet formation by human megakaryocytes. Haematologica 2012, 97, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Casella, I.; Feccia, T.; Chelucci, C.; Samoggia, P.; Castelli, G.; Guerriero, R.; Parolini, I.; Petrucci, E.; Pelosi, E.; Morsilli, O.; et al. Autocrine-paracrine VEGF loops potentiate the maturation of megakaryocytic precursors through Flt1 receptor. Blood 2003, 101, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Möhle, R.; Green, D.; Moore, M.A.; Nachman, R.L.; Rafii, S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc. Natl. Acad. Sci. USA 1997, 94, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.P.; Rauova, L.; Bailey, M.; Sola-Visner, M.C.; Kowalska, M.A.; Poncz, M. Platelet factor 4 is a negative autocrine in vivo regulator of megakaryopoiesis: Clinical and therapeutic implications. Blood 2007, 110, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Malara, A.; Abbonante, V.; di Buduo, C.A.; Tozzi, L.; Currao, M.; Balduini, A. The secret life of a megakaryocyte: Emerging roles in bone marrow homeostasis control. Cell. Mol. Life Sci. 2015, 72, 1517–1536. [Google Scholar] [CrossRef] [PubMed]
- Di Buduo, C.A.; Alberelli, M.A.; Glembostky, A.C.; Podda, G.; Lev, P.R.; Cattaneo, M.; Landolfi, R.; Heller, P.G.; Balduini, A.; de Candia, E. Abnormal proplatelet formation and emperipolesis in cultured human megakaryocytes from gray platelet syndrome patients. Sci. Rep. 2016, 6, 23213. [Google Scholar] [CrossRef] [PubMed]
- Balduini, C.L.; Pecci, A.; Noris, P. Diagnosis and management of inherited thrombocytopenias. Semin. Thromb. Hemost. 2013, 39, 161–171. [Google Scholar] [PubMed]
- Eto, K.; Kunishima, S. Linkage between the mechanisms of thrombocytopenia and thrombopoiesis. Blood 2016, 127, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.; Levine, R.F. The origin, development and regulation of megakaryocytes. Br. J. Haematol. 1982, 52, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Ru, Y.X.; Zhao, S.X.; Dong, S.X.; Yang, Y.Q.; Eyden, B. On the maturation of megakaryocytes: A review with original observations on human in vivo cells emphasizing morphology and ultrastructure. Ultrastruct. Pathol. 2015, 39, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Eckly, A.; Heijnen, H.; Pertuy, F.; Geerts, W.; Proamer, F.; Rinckel, J.Y.; Léon, C.; Lanza, F.; Gachet, C. Biogenesis of the demarcation membrane system (DMS) in megakaryocytes. Blood 2014, 123, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Lacabaratz-Porret, C.; Launay, S.; Corvazier, E.; Bredoux, R.; Papp, B.; Enouf, J. Biogenesis of endoplasmic reticulum proteins involved in Ca2+ signalling during megakaryocytic differentiation: An in vitro study. Biochem. J. 2000, 350, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Nurden, P.; Debili, N.; Vainchenker, W.; Bobe, R.; Bredoux, R.; Corvazier, E.; Combrie, R.; Fressinaud, E.; Meyer, D.; Nurden, A.T.; et al. Impaired megakaryocytopoiesis in type 2B von Willebrand disease with severe thrombocytopenia. Blood 2006, 108, 2587–2595. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Yamamoto-Hino, M.; Miyawaki, A.; Furuichi, T.; Mikoshiba, K.; Hasegawa, M. Subtypes of inositol 1,4,5-trisphosphate receptor in human hematopoietic cell lines: Dynamic aspects of their cell-type specific expression. FEBS Lett. 1994, 349, 191–196. [Google Scholar] [CrossRef]
- Berg, L.P.; Shamsher, M.K.; El-Daher, S.S.; Kakkar, V.V.; Authi, K.S. Expression of human TRPC genes in the megakaryocytic cell lines MEG01, DAMI and HEL. FEBS Lett. 1997, 403, 83–86. [Google Scholar] [CrossRef]
- Wakabayashi, I.; Marumo, M.; Graziani, A.; Poteser, M.; Groschner, K. TRPC4 expression determines sensitivity of the platelet-type capacitative Ca2+ entry channel to intracellular alkalosis. Platelets 2006, 17, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Den Dekker, E.; Molin, D.G.; Breikers, G.; van Oerle, R.; Akkerman, J.W.; van Eys, G.J.; Heemskerk, J.W. Expression of transient receptor potential mrna isoforms and Ca2+ influx in differentiating human stem cells and platelets. Biochim. Biophys. Acta 2001, 1539, 243–255. [Google Scholar] [CrossRef]
- Den Dekker, E.; Gorter, G.; van der Vuurst, H.; Heemskerk, J.W.; Akkerman, J.W. Biogenesis of G-protein mediated calcium signaling in human megakaryocytes. Thromb. Haemost. 2001, 86, 1106–1113. [Google Scholar] [PubMed]
- Putney, J.W.; Bird, G.S. Cytoplasmic calcium oscillations and store-operated calcium influx. J. Physiol. 2008, 586, 3055–3059. [Google Scholar] [CrossRef] [PubMed]
- Wedel, B.; Boyles, R.R.; Putney, J.W.; Bird, G.S. Role of the store-operated calcium entry proteins STIM1 and Orai1 in muscarinic cholinergic receptor-stimulated calcium oscillations in human embryonic kidney cells. J. Physiol. 2007, 579, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Shuttleworth, T.J. What drives calcium entry during [Ca2+]i oscillations?—Challenging the capacitative model. Cell Calcium 1999, 25, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.C.; Tonelli, F.M.; Vieira, A.L.; Kihara, A.H.; Ulrich, H.; Resende, R.R. Studying complex system: Calcium oscillations as attractor of cell differentiation. Integr. Biol. 2016, 8, 130–148. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liu, Y.; Lipsky, S.; Cho, M. Physical manipulation of calcium oscillations facilitates osteodifferentiation of human mesenchymal stem cells. FASEB J. 2007, 21, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Parkash, J.; Asotra, K. Calcium wave signaling in cancer cells. Life Sci. 2010, 87, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Heise, N.; Palme, D.; Misovic, M.; Koka, S.; Rudner, J.; Lang, F.; Salih, H.R.; Huber, S.M.; Henke, G. Non-selective cation channel-mediated Ca2+-entry and activation of Ca2+/calmodulin-dependent kinase II contribute to G2/M cell cycle arrest and survival of irradiated leukemia cells. Cell. Physiol. Biochem. 2010, 26, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, G.; Mannhalter, C. Increased expression of transient receptor potential canonical 6 (TRPC6) in differentiating human megakaryocytes. Cell Biol. Int. 2016, 40, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.N.; Tolhurst, G.; Walmsley, G.; Vizuete-Forster, M.; Miller, N.; Mahaut-Smith, M.P. Molecular and electrophysiological characterization of transient receptor potential ion channels in the primary murine megakaryocyte. J. Physiol. 2006, 576, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Varga-Szabo, D.; Authi, K.S.; Braun, A.; Bender, M.; Ambily, A.; Hassock, S.R.; Gudermann, T.; Dietrich, A.; Nieswandt, B. Store-operated Ca2+ entry in platelets occurs independently of transient receptor potential (TRP) C1. Pflugers Arch. 2008, 457, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, G.; Gupta, S.; Thielmann, I.; Pleines, I.; Varga-Szabo, D.; May, F.; Mannhalter, C.; Dietrich, A.; Nieswandt, B.; Braun, A. Defective diacylglycerol-induced Ca2+ entry but normal agonist-induced activation responses in TRPC6-deficient mouse platelets. J. Thromb. Haemost. 2012, 10, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Borst, O.; Schmidt, E.M.; Münzer, P.; Schönberger, T.; Towhid, S.T.; Elvers, M.; Leibrock, C.; Schmid, E.; Eylenstein, A.; Kuhl, D.; et al. The serum- and glucocorticoid-inducible kinase 1 (SGK1) influences platelet calcium signaling and function by regulation of Orai1 expression in megakaryocytes. Blood 2012, 119, 251–261. [Google Scholar] [CrossRef] [PubMed]
- López, E.; Berna-Erro, A.; Salido, G.M.; Rosado, J.A.; Redondo, P.C. FKBP52 is involved in the regulation of SOCE channels in the human platelets and MEG 01 cells. Biochim. Biophys. Acta 2013, 1833, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Kusakabe, M.; Sunadome, K.; Yamamoto, T.; Nishida, E. The kinase SGK1 in the endoderm and mesoderm promotes ectodermal survival by down-regulating components of the death-inducing signaling complex. Sci. Signal. 2011, 4, ra2. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Böhmer, C.; Palmada, M.; Seebohm, G.; Strutz-Seebohm, N.; Vallon, V. (Patho) physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol. Rev. 2006, 86, 1151–1178. [Google Scholar] [CrossRef] [PubMed]
- Borst, O.; Münzer, P.; Schmid, E.; Schmidt, E.M.; Russo, A.; Walker, B.; Yang, W.; Leibrock, C.; Szteyn, K.; Schmidt, S.; et al. 1,25(OH)2 vitamin D3-dependent inhibition of platelet Ca2+ signaling and thrombus formation in klotho-deficient mice. FASEB J. 2014, 28, 2108–2119. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Schmid, E.; Almilaji, A.; Shumilina, E.; Borst, O.; Laufer, S.; Gawaz, M.; Lang, F. Effect of TGFβ on calcium signaling in megakaryocytes. Biochem. Biophys. Res. Commun. 2015, 461, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Almilaji, A.; Yan, J.; Hosseinzadeh, Z.; Schmid, E.; Gawaz, M.; Lang, F. Up-regulation of Na+/Ca2+ exchange in megakaryocytes following TGFβ1 treatment. Cell. Physiol. Biochem. 2016, 39, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Björquist, A.; di Buduo, C.A.; Femia, E.A.; Storey, R.F.; Becker, R.C.; Balduini, A.; Nylander, S.; Cattaneo, M. Studies of the interaction of ticagrelor with the P2Y13 receptor and with P2Y13-dependent pro-platelet formation by human megakaryocytes. Thromb. Haemost. 2016, 116, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Mountford, J.C.; Melford, S.K.; Bunce, C.M.; Gibbins, J.; Watson, S.P. Collagen or collagen-related peptide cause [Ca2+]i elevation and increased tyrosine phosphorylation in human megakaryocytes. Thromb. Haemost. 1999, 82, 1153–1159. [Google Scholar] [PubMed]
- Kamal, T.; Green, T.N.; Morel-Kopp, M.C.; Ward, C.M.; McGregor, A.L.; McGlashan, S.R.; Bohlander, S.K.; Browett, P.J.; Teague, L.; During, M.J.; et al. Inhibition of glutamate regulated calcium entry into leukemic megakaryoblasts reduces cell proliferation and supports differentiation. Cell Signal. 2015, 27, 1860–1872. [Google Scholar] [CrossRef] [PubMed]
- Grosse, J.; Braun, A.; Varga-Szabo, D.; Beyersdorf, N.; Schneider, B.; Zeitlmann, L.; Hanke, P.; Schropp, P.; Mühlstedt, S.; Zorn, C.; et al. An EF hand mutation in STIM1 causes premature platelet activation and bleeding in mice. J. Clin. Investig. 2007, 117, 3540–3550. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A. Myeloproliferative neoplasms: A decade of discoveries and treatment advances. Am. J. Hematol. 2016, 91, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Norfo, R.; Pennucci, V.; Zini, R.; Manfredini, R. Genomic landscape of megakaryopoiesis and platelet function defects. Blood 2016, 127, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.; Kvasnicka, H.; Tefferi, A.; Barosi, G.; Orazi, A.; Vardiman, J. Primary myelofibrosis; Swerdlow, S.H., Campo, E., Harris, N.L., Jaffee, E.S., Pileri, S.A., Stein, H., Thiele, J., Vardiman, J.W., Eds.; IARC Press: Lyon, France, 2008; pp. 44–47. [Google Scholar]
- Balduini, A.; Badalucco, S.; Pugliano, M.T.; Baev, D.; de Silvestri, A.; Cattaneo, M.; Rosti, V.; Barosi, G. In vitro megakaryocyte differentiation and proplatelet formation in Ph-negative classical myeloproliferative neoplasms: Distinct patterns in the different clinical phenotypes. PLoS ONE 2011, 6, e21015. [Google Scholar] [CrossRef] [PubMed]
- Ciurea, S.O.; Merchant, D.; Mahmud, N.; Ishii, T.; Zhao, Y.; Hu, W.; Bruno, E.; Barosi, G.; Xu, M.; Hoffman, R. Pivotal contributions of megakaryocytes to the biology of idiopathic myelofibrosis. Blood 2007, 110, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Lasho, T.L.; Finke, C.M.; Knudson, R.A.; Ketterling, R.; Hanson, C.H.; Maffioli, M.; Caramazza, D.; Passamonti, F.; Pardanani, A. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: Clinical, cytogenetic and molecular comparisons. Leukemia 2014, 28, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Klampfl, T.; Gisslinger, H.; Harutyunyan, A.S.; Nivarthi, H.; Rumi, E.; Milosevic, J.D.; Them, N.C.; Berg, T.; Gisslinger, B.; Pietra, D.; et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N. Engl. J. Med. 2013, 369, 2379–2390. [Google Scholar] [CrossRef] [PubMed]
- Nangalia, J.; Massie, C.E.; Baxter, E.J.; Nice, F.L.; Gundem, G.; Wedge, D.C.; Avezov, E.; Li, J.; Kollmann, K.; Kent, D.G.; et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N. Engl. J. Med. 2013, 369, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Chachoua, I.; Pecquet, C.; El-Khoury, M.; Nivarthi, H.; Albu, R.I.; Marty, C.; Gryshkova, V.; Defour, J.P.; Vertenoeil, G.; Ngo, A.; et al. Thrombopoietin receptor activation by myeloproliferative neoplasm associated calreticulin mutants. Blood 2016, 127, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Marty, C.; Pecquet, C.; Nivarthi, H.; El-Khoury, M.; Chachoua, I.; Tulliez, M.; Villeval, J.L.; Raslova, H.; Kralovics, R.; Constantinescu, S.N.; et al. Calreticulin mutants in mice induce an MPL-dependent thrombocytosis with frequent progression to myelofibrosis. Blood 2016, 127, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Corbett, E.F.; Mesaeli, N.; Nakamura, K.; Opas, M. Calreticulin: One protein, one gene, many functions. Biochem. J. 1999, 344, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Pietra, D.; Rumi, E.; Ferretti, V.V.; Buduo, C.A.; Milanesi, C.; Cavalloni, C.; Sant′Antonio, E.; Abbonante, V.; Moccia, F.; Casetti, I.C.; et al. Differential clinical effects of different mutation subtypes in CALR-mutant myeloproliferative neoplasms. Leukemia 2016, 30, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Bastianutto, C.; Clementi, E.; Codazzi, F.; Podini, P.; de Giorgi, F.; Rizzuto, R.; Meldolesi, J.; Pozzan, T. Overexpression of calreticulin increases the Ca2+ capacity of rapidly exchanging Ca2+ stores and reveals aspects of their lumenal microenvironment and function. J. Cell Biol. 1995, 130, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Fasolato, C.; Pizzo, P.; Pozzan, T. Delayed activation of the store-operated calcium current induced by calreticulin overexpression in RBL-1 cells. Mol. Biol. Cell 1998, 9, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Longo, F.J.; Wintermantel, M.R.; Jiang, X.; Clark, R.A.; DeLisle, S. Calreticulin modulates capacitative Ca2+ influx by controlling the extent of inositol 1,4,5-trisphosphate-induced Ca2+ store depletion. J. Biol. Chem. 2000, 275, 36676–36682. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Buduo, C.A.; Balduini, A.; Moccia, F. Pathophysiological Significance of Store-Operated Calcium Entry in Megakaryocyte Function: Opening New Paths for Understanding the Role of Calcium in Thrombopoiesis. Int. J. Mol. Sci. 2016, 17, 2055. https://doi.org/10.3390/ijms17122055
Di Buduo CA, Balduini A, Moccia F. Pathophysiological Significance of Store-Operated Calcium Entry in Megakaryocyte Function: Opening New Paths for Understanding the Role of Calcium in Thrombopoiesis. International Journal of Molecular Sciences. 2016; 17(12):2055. https://doi.org/10.3390/ijms17122055
Chicago/Turabian StyleDi Buduo, Christian A., Alessandra Balduini, and Francesco Moccia. 2016. "Pathophysiological Significance of Store-Operated Calcium Entry in Megakaryocyte Function: Opening New Paths for Understanding the Role of Calcium in Thrombopoiesis" International Journal of Molecular Sciences 17, no. 12: 2055. https://doi.org/10.3390/ijms17122055
APA StyleDi Buduo, C. A., Balduini, A., & Moccia, F. (2016). Pathophysiological Significance of Store-Operated Calcium Entry in Megakaryocyte Function: Opening New Paths for Understanding the Role of Calcium in Thrombopoiesis. International Journal of Molecular Sciences, 17(12), 2055. https://doi.org/10.3390/ijms17122055