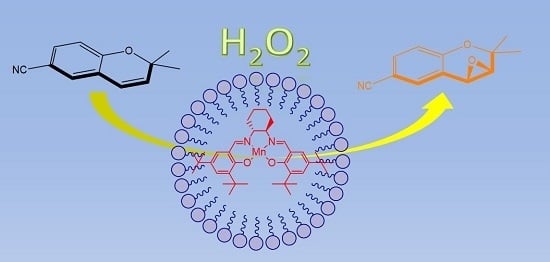
(Salen)Mn(III) Catalyzed Asymmetric Epoxidation Reactions by Hydrogen Peroxide in Water: A Green Protocol
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General
3.2. Preparation of Alkenes
3.3. Enantioselective Epoxidation in Surfactant Solutions
3.4. Product Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Srour, H.; Le Maux, P.; Chevance, S.; Simouneaux, G. Metal-catalyzed asymmetric sulfoxidation, epoxidation and hydroxylation by hydrogen peroxide. Coord. Chem. Rev. 2013, 257, 3030–3050. [Google Scholar] [CrossRef] [Green Version]
- De Faveri, G.; Ilyashenko, G.; Watkinson, M. Recent advances in catalytic asymmetric epoxidation using the environmentally benign oxidant hydrogen peroxide and its derivatives. Chem. Soc. Rev. 2011, 40, 1722–1760. [Google Scholar] [CrossRef] [PubMed]
- Lane, B.S.; Burgess, K. Metal-catalyzed epoxidations of alkenes with hydrogen peroxide. Chem. Rev. 2003, 103, 2457–2474. [Google Scholar] [CrossRef] [PubMed]
- McGarrigle, E.M.; Gilheany, D.G. Chromium- and manganese-salen promoted epoxidation of alkenes. Chem. Rev. 2005, 105, 1563–1602. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.-P.; Shi, Z.-H.; Li, N.-G.; Tang, Y.-P.; Li, W.; Tang, H.; Zhang, W.; Shen, M.-Z.; Duan, J.-A. Asymmetric epoxidation of olefins with homogeneous chiral (salen) manganese(III) complexes. Curr. Org. Chem. 2013, 17, 2936–2970. [Google Scholar] [CrossRef]
- Jing, J.; Chen, J.J.; Hai, Y.; Zhan, J.; Xu, P.; Zhang, J.L. Rational design of ZnSalen as a single and two photon activatable fluorophore in living cells. Chem. Sci. 2012, 3, 3315–3320. [Google Scholar] [CrossRef]
- Yang, S.; Kou, H.; Wang, H.; Cheng, K.; Wang, J. Efficient electrolyte of N,N’-bis(salicylidene) ethylenediamine zinc(II) iodide in dye-sensitized solar cells. New J. Chem. 2010, 34, 313–317. [Google Scholar] [CrossRef]
- Shoji, R.; Ikenomoto, S.; Sunaga, N.; Sugiyama, M.; Akitsu, T. Absorption wavelength extension for dye-sensitized solar cells by varying the substituents of chiral salen Cu(II) complexes. J. Appl. Sol. Chem. Mod. 2016, 5, 48–56. [Google Scholar]
- D’Urso, A.; Tudisco, C.; Ballistreri, F.; Condorelli, G.G.; Randazzo, R.; Tomaselli, G.A.; Toscano, R.M.; Trusso Sfrazzetto, G.; Pappalardo, A. Enantioselective extraction mediated by a chiral cavitand–salen covalently assembled on a porous silicon surface. Chem. Commun. 2014, 50, 4993–4996. [Google Scholar] [CrossRef] [PubMed]
- Tudisco, C.; Trusso Sfrazzetto, G.; Pappalardo, A.; Motta, A.; Tomaselli, G.A.; Fragalà, I.L.; Ballistreri, F.P.; Condorelli, G.G. Covalent functionalization of silicon surfaces with a cavitand–modified salen. Eur. J. Inorg. Chem. 2011, 13, 2124–2131. [Google Scholar] [CrossRef]
- Ballistreri, F.P.; Pappalardo, A.; Tomaselli, G.A.; Toscano, R.M.; Sfrazzetto, G.T. Heteroditopic chiral uranyl–salen receptor for molecular recognition of amino acid ammonium Salts. Eur. J. Org. Chem. 2010, 20, 3806–3810. [Google Scholar] [CrossRef]
- Amato, M.E.; Ballistreri, F.P.; D’Agata, S.; Pappalardo, A.; Tomaselli, G.A.; Toscano, R.M.; Sfrazzetto, G.A. Enantioselective molecular recognition of chiral organic ammonium ions and amino acids using cavitand–salen-based receptors. Eur. J. Org. Chem. 2011, 28, 5674–5680. [Google Scholar] [CrossRef]
- Pappalardo, A.; Amato, M.E.; Ballistreri, F.P.; Tomaselli, G.A.; Toscano, R.M.; Trusso Sfrazzetto, G. Pair of diastereomeric uranyl salen cavitands displaying opposite enantiodiscrimination of α-amino acid ammonium Salts. J. Org. Chem. 2012, 77, 7684–7687. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, E.N. Catalytic Asymmetric Synthesis, 1st ed.; Ojima, I., Ed.; VCH: Weinheim, Germany, 1993; pp. 287–325. [Google Scholar]
- Katsuki, T. Catalytic Asymmetric Synthesis, 2nd ed.; Ojima, I., Ed.; Wiley-VCH: New York, NY, USA, 2004; Chapter 6. [Google Scholar]
- Sfrazzetto, G.T.; Millesi, S.; Pappalardo, A.; Toscano, R.M.; Ballistreri, F.P.; Tomaselli, G.A.; Gulino, A. Olefin epoxidation by a (salen)Mn(III) catalyst covalently grafted on glass beads. Cat. Sci. Technol. 2015, 5, 673–679. [Google Scholar] [CrossRef]
- Amato, M.E.; Ballistreri, F.P.; Pappalardo, A.; Tomaselli, G.A.; Toscano, R.M.; Williams, D.J. Novel chiral (salen)MnIII complexes containing a calix[4]arene unit as catalysts for enantioselective epoxidation reactions of (Z)-aryl alkenes. Eur. J. Org. Chem. 2005, 16, 3562–3570. [Google Scholar] [CrossRef]
- Ballistreri, F.P.; Brinchi, L.; Germani, R.; Savelli, G.; Tomaselli, G.A.; Toscano, R.M. Enantioselective epoxidations of alkenes catalyzed by (salen)Mn(III) in aqueous surfactant systems. Tetrahedron 2008, 64, 10239–10243. [Google Scholar] [CrossRef]
- Brink, G.-J.; Arends, I.W.C.E.; Sheldon, R.A. The Baeyer-Villiger reaction: New developments toward greener procedures. Chem. Rev. 2004, 104, 4105–4123. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yamamoto, H. Asymmetric Epoxidation Using Hydrogen Peroxide as Oxidant. Chem. Asian J. 2015, 10, 2056–2068. [Google Scholar] [CrossRef] [PubMed]
- Colladon, M.; Scarso, A.; Sgarbossa, P.; Michelin, R.A.; Strukul, G. Regioselectivity and diasteroselectivity in Pt(II)-mediated “green” catalytic epoxidation of terminal alkenes with hydrogen peroxide: Mechanistic insight into a peculiar substrate selectivity. J. Am. Chem. Soc. 2007, 129, 7680–7689. [Google Scholar] [CrossRef] [PubMed]
- Salem, I.A.; El-Sheikh, M.Y.; Zaki, A.B. Kinetics and mechanism of the decomposition of hydrogen peroxide catalyzed by Mn(II)-bis-salicylaldimine complexes. Monatsh. Chem. 1995, 126, 393–399. [Google Scholar] [CrossRef]
- Baleizao, C.; Garcia, H. Chiral salen complexes: An overview to recoverable and reusable homogeneous and heterogeneous catalysts. Chem. Rev. 2006, 106, 3987–4043. [Google Scholar] [CrossRef] [PubMed]
- Pietikainen, P. Convenient asymmetric (salen)Mn(III)-catalyzed epoxidation of unfunctionalized alkenes with hydrogen peroxide using carboxylate salt cocatalysts. Tetrahedron 1998, 54, 4319–4326. [Google Scholar] [CrossRef]
- Watanabe, Y.; Namba, A.; Umezawa, N.; Kawahata, M.; Yamaguchi, K.; Higuchi, T. Enhanced catalase-like activity of manganese salen complexes in water: Effect of a three-dimensionally fixed auxiliary. Chem. Commun. 2006, 47, 4958–4960. [Google Scholar] [CrossRef] [PubMed]
- Grigoropoulou, G.; Clark, J.H.; Elings, J.A. Recent developments on the epoxidation of alkenes using hydrogen peroxide as an oxidant. Green Chem. 2003, 5, 1–7. [Google Scholar] [CrossRef]
- Irie, R.; Hosoya, N.; Katsuki, T. Enantioselective epoxidation of chromene derivatives using hydrogen peroxide as a terminal oxidant. Synlett 1994, 4, 255–256. [Google Scholar] [CrossRef]
- Schwenkreis, T.; Berkessel, A. A biomimetic catalyst for the asymmetric epoxidation of unfunctionalized olefins with hydrogen peroxide. Tetrahedron Lett. 1993, 34, 4785–4788. [Google Scholar] [CrossRef]
- Kureshy, R.I.; Khan, N.H.; Abdi, S.H.R.; Singh, S.; Ahmed, I.; Shukla, Ram S.; Jasra, R.V. Chiral Mn(III) salen complex-catalyzed enantioselective epoxidation of nonfunctionalized alkenes using urea-H2O2 adduct as oxidant. J. Catal. 2003, 219, 1–7. [Google Scholar] [CrossRef]
- Matsumoto, K.; Oguma, T.; Katsuki, T. Highly enantioselective epoxidation of styrenes catalyzed by proline-derived C1-symmetric titanium(salan) complexes. Angew. Chem. Int. Ed. 2009, 48, 7432–7435. [Google Scholar] [CrossRef] [PubMed]
- Feichtinger, D.; Plattner, D.A. Direct proof for O: MnV(salen) complexes. Angew. Chem. Int. Ed. 1997, 36, 1718–1719. [Google Scholar] [CrossRef]
- La Paglia Fragola, V.; Lupo, F.; Pappalardo, A.; Sfrazzetto, G.T.; Toscano, R.M.; Ballistreri, F.P.; Tomaselli, G.A.; Gulino, A. A surface-confined O: MnV(salen) oxene catalyst and high turnover values in asymmetric epoxidation of unfunctionalized olefins. J. Mater. Chem. 2012, 22, 20561–20565. [Google Scholar] [CrossRef]
- Battioni, P.; Renaud, J.P.; Bartoli, J.F.; Reina-Artiles, M.; Fort, M.; Mansuy, D.J. Monooxygenase-like oxidation of hydrocarbons by hydrogen peroxide catalyzed by manganese porphyrins and imidazole: Selection of the best catalytic system and nature of the active oxygen species. J. Am. Chem. Soc. 1988, 110, 8462–8470. [Google Scholar] [CrossRef]
- Anelli, P.L.; Banfi, S.; Montanari, F.; Quilici, S. Synergistic effect of lipophilic carboxylic acids and heterocyclic axial ligands in alkene epoxidation by hydrogen peroxide catalyzed by manganese(III) tetraarylporphyrins. J. Chem. Soc. Chem. Commun. 1989, 779–780. [Google Scholar] [CrossRef]
- Banfi, S.; Maiocchi, A.; Montanari, F.; Quici, S. Hydrogen peroxide epoxidation of olefins catalyzed by manganese(III) tetraarylporphyrins. Synergistic effect of carboxylic acids and of lipophilic heterocyclic axial ligands. Gazz. Chim Ital. 1990, 120, 123–130. [Google Scholar]
- Gonsalves, A.M.D.A.; Johnstone, R.A.W.; Pereira, M.M.; Shaw, J. Metal-assisted reactions. Part 21: Epoxidation of alkenes catalyzed by manganese-porphyrins: The effects of various oxidatively-stable ligands and bases. J. Chem. Soc. Perkin Trans. 1991, 3, 645–649. [Google Scholar] [CrossRef]
- Shitama, H.; Katsuki, T. Asymmetric epoxidation using aqueous hydrogen peroxide as oxidant: Bio-inspired construction of pentacoordinated Mn-salen complexes and their catalysis. Tetrahedron Lett. 2006, 47, 3203–3207. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Nocera, D.G. A simple and versatile method for alkene epoxidation using aqueous hydrogen peroxide and manganese salophen catalysts. Tetrahedron Lett. 2006, 47, 1923–1924. [Google Scholar] [CrossRef]
- Finney, N.S.; Pospil, P.J.; Chang, S.; Palucki, M.; Konsler, R.G.; Hansen, K.B.; Jacobsen, E.N. On the viability of oxametallacyclic intermediates in the (salen)Mn-catalyzed asymmetric epoxidation. Angew. Chem. Int. Ed. 1997, 36, 1720–1723. [Google Scholar] [CrossRef]
- Senanayake, C.H.; Smith, G.B.; Ryan, K.M.; Fredenburgh, L.E.; Liu, J.; Roberts, F.E.; Hughes, D.L.; Larsen, R.D.; Verhoeven, T.R.; Reider, P.J. The role of 4-(3-phenylpropyl)pyridine N-oxide (P3NO) in the manganese-salen-catalyzed asymmetric epoxidation of indene. Tetrahedron Lett. 1996, 37, 3271–3274. [Google Scholar] [CrossRef]
- Hughes, D.L.; Smith, G.B.; Liu, J.; Dezeny, G. C.; Senanayake, C.K.; Larsen, R.D.; Verhoeven, T.R.; Reider, P.J. Mechanistic study of the jacobsen asymmetric epoxidation of indene. J. Org. Chem. 1997, 62, 2222–2229. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liang, L.; Weck, M. Micelle-based nanoreactors containing Ru-porphyrin for the epoxidation of terminal olefins in water. J. Mol. Catal. A: Chem. 2016, 417, 122–125. [Google Scholar] [CrossRef]
- Scheurer, A.; Mosset, P.; Spiegel, M.; Saalfrank, R.W. Reverse asymmetric catalytic epoxidation of unfunctionalized alkenes. Tetrahedron 1999, 55, 1063–1078. [Google Scholar] [CrossRef]
- Patti, A.; Pedotti, S.; Ballistreri, F.P.; Sfrazzetto, G.T. Synthesis and characterization of some chiral metal-salen complexes bearing a ferrocenophane substituent. Molecules 2009, 14, 4312–4325. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, G.M.; Thompson, A.L.; Ballistreri, F.P.; Pappalardo, A.; Sfrazzetto, G.T.; Tomaselli, G.A.; Toscano, R.M.; Punzo, F. An integrated X-ray and molecular dynamics study of uranyl-salen structures and properties. Dalton Trans. 2012, 41, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, R.; Gericke, R. Synthesis and antihypertensive activity of 4-(1,2-dihydro-2-oxo-1-pyridyl)-2H-1-benzopyrans and related compounds, new potassium channel activators J. Med. Chem. 1990, 33, 492–504. [Google Scholar] [CrossRef]
- Foltz, C.M.; Witkop, B.J. Stereochemistry of the 1-phenyl-1,2-propanediols and of α-isoephedrine. J. Am. Chem. Soc. 1957, 79, 201–205. [Google Scholar] [CrossRef]


| Entry | [AOE-14] (M) | Time (min) | Conv. b (%) | e.e. b (%) |
|---|---|---|---|---|
| 1 | 0.1 | 1 | 7 | 84 |
| 2 | 0.1 | 3 | 12 | 81 |
| 3 | 0.1 | 10 | 35 | 80 |
| 4 | 0.1 | 20 | 58 | 82 |
| 5 | 0.1 | 300 | 55 | 83 |
| 6 c | 0.1 | 10 d | 81 | 84 |
| 7 | 0.2 | 10 | 60 | 83 |
| 8 | 0.2 | 20 | 81 | 83 |
| 9 | 0.2 | 300 | 81 | 82 |
| Entry | Alkene | Conv. d (%) | e.e. b (%) | Conf. c |
|---|---|---|---|---|
| 10 | 6-CN-2,2-dimethylchromene | 81 e | 83 b,d | 3R,4R |
| 11 | 6-NO2-2,2-dimethylchromene | 61 | 86 | 3R,4R |
| 12 | 6-H-2,2-dimethylchromene | 87 | 95 | 3R,4R |
| 13 | 6-CH3-2,2-dimethylchromene | 78 | 80 | 3R,4R |
| Entry | Alkene | [Cat.] (10−3 M) | [AOE-14] (M) | Conv. b (%) | e.e. b (%) | Ratio d |
|---|---|---|---|---|---|---|
| 14 | cis-β-methylstyrene | 2 | 0.2 | 50 | 80 c | 100 |
| 15 | cis-β-methylstyrene | 4 | 0.6 | 70 | 80 c | 150 |
| 16 | cis-β-methylstyrene | 4 | 0.8 | 73 | 80 c | 200 |
| 17 | 1,2-dihydronaphthalene | 2 | 0.2 | 44 | 54 | 100 |
| 18 | 1,2-dihydronaphthalene | 4 | 0.6 | 92 | 54 | 150 |
| 19 | 1,2-dihydronaphthalene | 4 | 0.8 | 100 e | 54 | 200 |
| 20 | indene | 2 | 0.2 | 30 | 80 | 100 |
| 21 | indene | 4 | 0.6 | 66 | 80 | 150 |
| 22 | 2-methylindene | 1 | 0.2 | 25 | 90 | 50 |
| 23 | 2-methylindene | 2 | 0.6 | 51 | 91 | 100 |
| 24 | 2-methylindene | 4 | 0.8 | 87 | 91 | 200 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballistreri, F.P.; Gangemi, C.M.A.; Pappalardo, A.; Tomaselli, G.A.; Toscano, R.M.; Trusso Sfrazzetto, G. (Salen)Mn(III) Catalyzed Asymmetric Epoxidation Reactions by Hydrogen Peroxide in Water: A Green Protocol. Int. J. Mol. Sci. 2016, 17, 1112. https://doi.org/10.3390/ijms17071112
Ballistreri FP, Gangemi CMA, Pappalardo A, Tomaselli GA, Toscano RM, Trusso Sfrazzetto G. (Salen)Mn(III) Catalyzed Asymmetric Epoxidation Reactions by Hydrogen Peroxide in Water: A Green Protocol. International Journal of Molecular Sciences. 2016; 17(7):1112. https://doi.org/10.3390/ijms17071112
Chicago/Turabian StyleBallistreri, Francesco Paolo, Chiara M. A. Gangemi, Andrea Pappalardo, Gaetano A. Tomaselli, Rosa Maria Toscano, and Giuseppe Trusso Sfrazzetto. 2016. "(Salen)Mn(III) Catalyzed Asymmetric Epoxidation Reactions by Hydrogen Peroxide in Water: A Green Protocol" International Journal of Molecular Sciences 17, no. 7: 1112. https://doi.org/10.3390/ijms17071112
APA StyleBallistreri, F. P., Gangemi, C. M. A., Pappalardo, A., Tomaselli, G. A., Toscano, R. M., & Trusso Sfrazzetto, G. (2016). (Salen)Mn(III) Catalyzed Asymmetric Epoxidation Reactions by Hydrogen Peroxide in Water: A Green Protocol. International Journal of Molecular Sciences, 17(7), 1112. https://doi.org/10.3390/ijms17071112