Overview of Biological, Epidemiological, and Clinical Evidence of Radiation Hormesis
Abstract
:1. Introduction
2. LNT Hypothesis
3. Hormesis as a Universal Phenomenon
4. Biological Studies of Radiation Hormesis
4.1. Overview
4.2. Radioadaptive Response
4.3. Growth Promotion and Lifespan Elongation by Low-Dose Radiation
4.4. Suppression of Tumorigenesis and Metastasis by Low-Dose Radiation
4.5. Changes in Biochemical and Immunological Parameters Following Low Dose Radiation
5. Epidemiological and Human Studies
5.1. Cancer Incidence in Atomic Bomb Survivors
5.2. Lifespan and Cancer Mortality/Incidence by Low-Level Radiation Exposure
5.3. Effects of Radiation from Computed Tomography
5.4. Randomized Human Studies on the Effects of Low-Dose-Radiation-Emitting Mats
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LNT | Linear-no-threshold |
| SOD | Superoxide dismutase |
| CT | Computed tomography |
References
- Luckey, T.D.; Lawrence, K.S. Radiation hormesis; the good, the bad, and the ugly. Dose-Response 2006, 4, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Pollycove, M.; Feinendegen, L.E. Biologic responses to low doses of ionizing radiation: Detriment versus hormesis. Part 2. Dose responses of organisms. J. Nucl. Med. 2001, 42, 26N–32N. [Google Scholar] [PubMed]
- Calabrese, E.J.; O’Connor, M.K. Estimating risk of low radiation doses—A critical review of the BEIR VII report and its use of the linear no-threshold (LNT) hypothesis. Radiat. Res. 2014, 182, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, J.; Grantham, V. Radiation hormesis: Historical and current perspective. J. Nucl. Med. Technol. 2015, 43, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Sacks, B.; Meyerson, G.; Siegel, J.A. Epidemiology without biology: False paradigms, unfounded assumptions, and specious statistics in radiation science (with commentaries by Inge Schmitz-Feuerhake and Christopher Busby and a reply by the authors). Biol. Theory 2016, 11, 69–101. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.J. Artificial transmutation of the gene. Science 1927, 66, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. On the origins of the linear no-threshold (LNT) dogma by means of untruths, artful dodges and blind faith. Environ. Res. 2015, 142, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Caspari, E.; Stern, C. The influence of chronic irradiation with gamma rays at low dosages on the mutation rate in Drosophila melanogaster. Genetics 1948, 33, 75–95. [Google Scholar] [PubMed]
- Marcus, C.S. Destroying the linear no-threshold basis for radiation regulation: A commentary. Dose-Response 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. The mistaken birth and adoption of LNT: An abridged version. Dose-Response 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Sacks, B.; Siegel, J.A. Preserving the anti-scientific linear no-threshold myth: Authority, agnosticism, transparency, and the standard of care. Dose-Response 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Koana, T.; Okada, M.O.; Ogura, K.; Tsujimura, H.; Sakai, K. Reduction of background mutations by low-dose X irradiation of Drosophilia spermatocytes at a low dose rate. Radiat. Res. 2007, 167, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Koana, T.; Takashima, Y.; Okada, M.O.; Ikehata, M.; Miyakoshi, J.; Sakai, K. A threshold exists in the dose-response relationship for somatic mutation frequency induced by X irradiation of Drosophila. Radiat. Res. 2004, 161, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Hooker, A.M.; Bhat, M.; Day, T.K.; Lane, J.M.; Swinburne, S.J.; Morley, A.A.; Sykes, P.J. The linear no-threshold model does not hold for low-dose ionizing radiation. Radiat. Res. 2004, 162, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Tubiana, M.; Feinendegen, L.E.; Yang, C.; Kaminski, J.M. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology 2009, 251, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J.; Sachs, R.K. Estimating radiation-induced cancer risks at very low doses: Rationale for using a linear no-threshold approach. Radiat. Environ. Biophys. 2006, 44, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Teeguarden, J.D.; Dragan, Y.; Pitot, H.C. Hazard assessment of chemical carcinogens: The impact of hormesis. J. Appl. Toxicol. 2000, 20, 113–120. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Environmental hormesis and its fundamental biological basis: Rewriting the history of toxicology. Environ. Res. 2018, 165, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Lee, Y.M.; Choi, J.Y.; Jacobs, D.R., Jr.; Lee, D.H. Evolutionarily adapted hormesis-inducing stressors can be a practical solution to mitigate harmful effects of chronic exposure to low dose chemical mixtures. Environ. Pollut. 2018, 233, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Castillo, H.; Schoderbek, D.; Dulal, S.; Escobar, G.; Wood, J.; Nelson, R.; Smith, G. Stress induction in the bacteria Shewanella oneidensis and Deinococcus radiodurans in response to below-background ionizing radiation. Int. J. Radiat. Biol. 2015, 91, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.R.; Loke, W.K.; Khoo, B.C. Low-dose or low-dose-rate ionizing radiation-induced bioeffects in animal models. J. Radiat. Res. 2017, 58, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Alessio, N.; Del Gaudio, S.; Capasso, S.; Di Bernardo, G.; Cappabianca, S.; Cipollaro, M.; Peluso, G.; Galderisi, U. Low dose radiation induced senescence of human mesenchymal stromal cells and impaired the autophagy process. Oncotarget 2015, 6, 8155–8166. [Google Scholar] [CrossRef] [PubMed]
- Squillaro, T.; Galano, G.; de Rosa, R.; Peluso, G.; Galderisi, U. Concise Review: The effect of low-dose ionizing radiation on stem cell biology: A contribution to radiation risk. Stem Cells 2018. [CrossRef] [PubMed]
- Olivieri, G.; Bodycote, J.; Wolff, S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science 1984, 223, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Tapio, S.; Jacob, V. Radioadaptive response revisited. Radiat. Environ. Biophys. 2007, 46, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nenoi, M.; Wang, B.; Vares, G. In vivo radioadaptive response: A review of studies relevant to radiation-induced cancer risk. Hum. Exp. Toxicol. 2015, 34, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, A.; Shibamoto, Y.; Sugie, C.; Ito, M.; Ayakawa, S. Absence of radioadaptive responses in four cell-lines in vitro as determined by colony formation assay. Kurume Med. J. 2006, 53, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sugie, C.; Nakashima, M.; Kondo, T.; Iwata, H.; Shibamoto, Y. Changes in proliferation rate and plating efficiency and adaptive response during and after continuous low-dose-rate irradiation in cultured cells. Manuscript in preparation.
- Ito, M.; Shibamoto, Y.; Ayakawa, S.; Tomita, N.; Sugie, C.; Ogino, H. Low-dose whole-body irradiation induced radioadaptive response in C57BL/6 mice. J. Radiat. Res. 2007, 48, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, M.; Takeda, A.; Misonoh, J. Acquired radioresistance after low dose X-irradiation in mice. J. Radiat. Res. 1990, 31, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Feinendegen, L.E. Quantification of adaptive protection following low-dose irradiation. Health Phys. 2016, 110, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, K. Beneficial effects of low-dose radiation on human health and possibility for application to medicine. Acad. Trends 2011, 11, 75–79. [Google Scholar]
- Moskalev, A.A.; Plyusnina, E.N.; Shaposhnikov, M.V. Radiation hormesis and radioadaptive response in Drosophila melanogaster flies with different genetic backgrounds: The role of cellular stress-resistance mechanisms. Biogerontology 2011, 12, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Zhikrevetskaya, S.; Peregudova, D.; Danilov, A.; Plyusnina, E.; Krasnov, G.; Dmitriev, A.; Kudryavtseva, A.; Shaposhnikov, M.; Moskalev, A. Effect of low doses (5–40 cGy) of gamma-irradiation on lifespan and stress-related genes expression profile in Drosophilia melanogaster. PLoS ONE 2015, 10, e0133840. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Seong, K.M.; Lee, B.S.; Lee, I.K.; Yang, K.H.; Kim, J.Y.; Nam, S.Y. Chronic low-dose γ-irradiation of Drosophila melanogaster larvae induces gene expression changes and enhances locomotive behavior. J. Radiat. Res. 2015, 56, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Yusifov, N.I.; Kuzin, A.M.; Agaev, F.A.; Alieva, S.G. The effect of low level ionizing radiation on embryogenesis of silkworm, Bombyx mori L. Radiat. Environ. Biophys. 1990, 29, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Araujo Sde, S.; Paparella, S.; Dondi, D.; Bentivoglio, A.; Carbonera, D.; Balestrazzi, A. Physical methods for seed invigoration: Advents and challenges in seed technology. Front. Plant Sci. 2016, 7, 646. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, T.; Hotta, T.; Watanabe, I. Growth promotion of tomato and radish plants by solar UV radiation reaching the Earth’s surface. J. Photochem. Photobiol. B Biol. 1993, 19, 61–66. [Google Scholar] [CrossRef]
- Hajnorouzi, A.; Vaezzadeh, M.; Ghanati, F.; Jamnezhad, H.; Nahidian, B. Growth promotion and a decrease of oxidative stress in maize seedlings by a combination of geomagnetic and weak electromagnetic fields. J. Plant Physiol. 2011, 168, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Shibamoto, Y.; Kamei, Y.; Kamei, K.; Tsuchiya, T.; Aoyama, N. Continuous low-dose-rate irradiation promotes growth of silkworms. Dose-Response 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Ducoff, H.S. Form of the increased longevity of Tribolium after X-irradiation. Exp. Geront. 1975, 10, 189–193. [Google Scholar] [CrossRef]
- Caratero, A.; Courtade, M.; Bonnet, L.; Planel, H.; Caratero, C. Effect of a continuous gamma irradiation at a very low dose on the life span of mice. Gerontology 1998, 44, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Courtade, M.; Billote, C.; Gasset, G.; Caratero, A.; Charlet, J.P.; Pipy, B.; Caratero, C. Life span, cancer and non-cancer diseases in mouse exposed to a continuous very low dose of gamma-irradiation. Int. J. Radiat. Biol. 2002, 78, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, Y.; Sakamoto, K. Suppressive effect of low dose total body irradiation on lung metastasis: Dose dependency and effective period. Radiother. Oncol. 1993, 26, 177–179. [Google Scholar] [CrossRef]
- Sakai, K.; Hoshi, Y.; Nomura, T.; Oda, T.; Iwasaki, T.; Fujita, K.; Yamada, T.; Tanooka, H. Suppression of carcinogenic processes in mice by chronic low dose rate gamma-irradiation. Int. J. Low Radiat. 2003, 1, 142–146. [Google Scholar] [CrossRef]
- Ina, Y.; Tanooka, H.; Yamada, T.; Sakai, K. Suppression of thymic lymphoma induction by life-long low-dose-rate irradiation accompanied by immune activation in C57BL/6 mice. Radiat. Res. 2005, 163, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Cheda, A.; Wrembel-Wargocka, J.; Lisiak, E.; Nowosielska, E.M.; Marciniak, M.; Janiak, M.K. Single low doses of X rays inhibit the development of experimental tumor metastases and trigger the activities of NK cells in mice. Radiat. Res. 2004, 161, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Hosoi, Y.; Yamada, S.; Ono, T.; Sakamoto, K. Decreased incidence of thymic lymphoma in AKR mice as a result of chronic, fractionated low-dose total-body X irradiation. Radiat. Res. 1996, 146, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Myojin, M.; Hosoi, Y.; Ogawa, Y.; Nemoto, K.; Takai, Y.; Kakuto, Y.; Yamada, S.; Watabe, N. Fundamental and clinical studies on cancer control with total or upper half body irradiation. J. Jpn. Soc. Ther. Radiol. Oncol. 1997, 9, 161–175. [Google Scholar]
- Upton, A.C. Radiation hormesis: Data and interpretations. Crit. Rev. Toxicol. 2001, 31, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Shibamoto, Y.; Ayakawa, S.; Tomita, N.; Sugie, C.; Ogino, H. Effect of low-dose total-body irradiation on transplantability of tumor cells in syngeneic mice. J. Radiat. Res. 2008, 49, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Lemon, J.A.; Phan, N.; Boreham, D.R. Single CT scan prolongs survival by extending cancer latency in Trp53 heterozygous mice. Radiat. Res. 2017, 188, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Lemon, J.A.; Phan, N.; Boreham, D.R. Multiple CT scans extend lifespan by delaying cancer progression in cancer-prone mice. Radiat. Res. 2017, 188, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.; Kim, M.J.; Kim, R.K.; Kaushik, N.K.; Seong, K.M.; Nam, S.Y.; Lee, S.J. Low-dose radiation decreases tumor progression via the inhibition of the JAK1/STAT3 signaling axis in breast cancer cell lines. Sci. Rep. 2017, 7, 43361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.S.; Moore, J.E.; Walb, M.C.; Kock, N.D.; Attia, A.; Isom, S.; McBride, J.E.; Munley, M.T. Chemoprevention by N-acetylcysyrin of low-dose CT-induced murine lung tumorigenesis. Carcinogenesis 2013, 34, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Koana, T.; Tauchi, H.; Sakai, K. Activation of antioxidative enzymes induced by low-dose-rate whole-body γ irradiation: Adaptive response in terms of initial DNA damage. Radiat. Res. 2006, 166, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, K.; Kojima, S.; Takahashi, M.; Nomura, T.; Iriyama, K. Change of glutathione peroxidase synthesis along with that of superoxide dismutase synthesis in mice spleens after low-dose X-ray irradiation. Biochim. Biophys. Acta 1998, 1381, 265–270. [Google Scholar] [CrossRef]
- Kataoka, T. Study of antioxidative effects and anti-inflammatory effects in mice due to low-dose X-irradiation or radon inhalation. J. Radiat. Res. 2013, 54, 587–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Large, M.; Hehlgans, S.; Reichert, S.; Gaipl, U.S.; Fournier, C.; Rödel, C.; Weiss, C.; Rödel, F. Study of the anti-inflammatory effects of low-dose radiation: The contribution of biphasic regulation of the antioxidative system in endothelial cells. Strahlenther. Onkol. 2015, 191, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Vilenchik, M.M.; Knudson, A.G., Jr. Inverse radiation dose-rate effects on somatic and germ-line mutations and DNA damage rates. Proc. Natl. Acad. Sc. USA 2000, 97, 5381–5386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Hofe, E.; Kennedy, A.R. X-ray induction of O6-alkylguanine-DNA alkyltransferase protects against some of the biological effects of N-methyl-N′-nitro-N-nitrosoguanidine in C3H 10T1/2 cells. Radiat. Res. 1991, 127, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Ina, Y.; Sakai, K. Activation of immunological network by chronic low-dose-rate irradiation in wild-type mouse strains: Analysis of immune cell populations and surface molecules. Int. J. Radiat. Biol. 2005, 81, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, A.; Adachi, Y.; Koike-Kiriyama, N.; Suzuki, Y.; Iwasaki, M.; Koike, Y.; Nakano, K.; Mukaide, H.; Imamura, M.; Ikehara, S. Effects of low-dose irradiation on enhancement of immunity by dendritic cells. J. Radiat. Res. 2007, 48, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Nowosielska, E.M.; Wrembel-Wargocka, J.; Cheda, A.; Lisiak, E.; Janiak, M.K. Enhanced cytotoxic activity of macrophages and suppressed tumor metastases in mice irradiated with low doses of X-rays. J. Radiat. Res. 2006, 47, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, K.; Mitsunobu, F.; Kojima, S.; Shibakura, M.; Kataoka, T.; Hanamoto, K.; Tanizaki, Y. The elevation of p53 protein levels and SOD activity in the resident blood of the Misasa radon hot spring district. J. Radiat. Res. 2005, 46, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xiong, S.; Zhang, L.; Chu, Y. Enhancement of antitumor immunity by low-dose total body irradiationis associated with selectively decreasing the proportion and number of T regulatory cells. Cell. Mol. Immunol. 2010, 7, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Ozasa, K.; Shimizu, Y.; Suyama, A.; Kasagi, F.; Soda, M.; Grant, E.J.; Sakata, R.; Sugiyama, H.; Kodama, K. Studies of the mortality of atomic bomb survivors, Report 14, 1950–2003: An overview of cancer and noncancer diseases. Radiat. Res. 2012, 177, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Grant, E.J.; Brenner, A.; Sugiyama, H.; Sakata, R.; Sadakane, A.; Utada, M.; Cahoon, E.K.; Milder, C.M.; Soda, M.; Cullings, H.M.; et al. Solid cancer incidence among the life span study of atomic bomb survivors: 1958–2009. Radiat. Res. 2017, 187, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, W.F.; Paretzke, H.G.; Jacob, P. No evidence for increased tumor rates below 200 mSv in the atomic bomb survivors data. Radiat. Environ. Biophys. 1997, 36, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Pierce, D.A.; Preston, D.L. Radiation-related cancer risks at low doses among atomic bomb survivors. Radiat. Res. 2000, 154, 178–186. [Google Scholar] [CrossRef]
- Doss, M. Evidence supporting radiation hormesis in atomic bomb survivor cancer mortality data. Dose Response 2012, 10, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Sutou, S. Rediscovery of an old article reporting that the area around the epicenter in Hiroshima was heavily contaminated with residual radiation, indicating that exposure doses of A-bomb survivors were largely underestimated. J. Radiat. Res. 2017, 58, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Okajima, S.; Fujita, S.; Harley, J.H. Radiation doses from residual radioactivity. In US-Japan Joint Reassessment of Atomic Bomb Radiation Dosimetry in Hiroshima and Nagasaki; Final Report; Roesch, W.C., Ed.; Radiation Effects Research Foundation: Hiroshima, Japan, 1987; Volume 1, pp. 205–226. [Google Scholar]
- Yokota, K.; Mine, M.; Kondo, H.; Matsuda, N.; Shibata, Y.; Takamura, N. Cancer mortality in residents of the terrain-shielded are exposed to fallout from the Nagasaki atomic bombing. J. Radiat. Res. 2018, 59, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nambi, K.S.; Soman, S.D. Environmental radiation and cancer in India. Health Phys. 1987, 52, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Mifune, M.; Sobue, T.; Arimoto, H.; Komoto, Y.; Kondo, S.; Tanooka, H. Cancer mortality survey in a spa area (Misasa, Japan) with a high radon background. Jpn. J. Cancer Res. 1992, 83, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Honjo, S.; Kawamura, H.; Koishi, F.; Suzuki, T.; Hirohata, T. Cancer mortality in low radon spa area. Jpn. J. Cancer Res. 1994, 85, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Sobue, T.; Lee, V.S.; Tanooka, H.; Mifune, M.; Suyama, A.; Koga, T.; Morishima, H.; Kondo, S. Mortality and cancer incidence in Misasa, Japan, a spa area with elevated radon levels. Jpn. J. Cancer Res. 1998, 89, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, K.; Okayama University, Okayama, Japan. Incidence of various cancers in the Misasa spa area. Personal communication, 2018. [Google Scholar]
- Cohen, B.L. Test of the linear-no threshold theory of radiation carcinogenesis for inhaled radon decay products. Health Phys. 1995, 68, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Luan, Y.C.; Shieh, M.C.; Chen, S.T.; Kung, H.T.; Soong, K.L.; Yeh, Y.C.; Chou, T.S.; Mong, S.H.; Wu, J.T.; et al. Effects of cobalt-60 exposure on health of Taiwan residents suggest new approach needed in radiation protection. Dose Response 2006, 25, 63–75. [Google Scholar] [CrossRef]
- Hwang, S.L.; Hwang, J.S.; Yang, Y.T.; Hsieh, W.A.; Chang, T.C.; Guo, H.R.; Tsai, M.H.; Tang, J.L.; Lin, I.F.; Chang, W.P. Estimates of relative risks for cancers in a population after prolonged low-dose rate radiation exposure: A follow-up assessment from 1983 to 2005. Radiat. Res. 2008, 170, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Cardis, E.; Vrijheid, M.; Blettner, M.; Gilbert, E.; Hakama, M.; Hill, C.; Howe, G.; Kaldor, J.; Muirhead, C.R.; Schubauer-Berigan, M.; et al. Risk of cancer after low doses of ionising radiation: Retrospective cohort study in 15 countries. BMJ 2005, 331, 77. [Google Scholar] [CrossRef] [PubMed]
- Muirhead, C.R.; O’Hagan, J.A.; Haylock, R.G.; Phillipson, M.A.; Willcock, T.; Berridge, G.L.; Zhang, W. Mortality and cancer incidence following occupational radiation exposure: Third analysis of the National Registry for Radiation Workers. Br. J. Cancer 2009, 100, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Leuraud, K.; Richardson, D.B.; Cardis, E.; Daniels, R.D.; Gillies, M.; O’Hagan, J.A.; Hamra, G.B.; Haylock, R.; Laurier, D.; Moissonnier, M.; et al. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): An international cohort study. Lancet Haematol. 2015, 2, e276–e281. [Google Scholar] [CrossRef]
- Richardson, D.B.; Cardis, E.; Daniels, R.D.; Gillies, M.; O’Hagan, J.A.; Hamra, G.B.; Haylock, R.; Laurier, D.; Leuraud, K.; Moissonnier, M.; et al. Risk of cancer from occupational exposure to ionising radiation: Retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS). BMJ 2015, 351, h5359. [Google Scholar] [CrossRef] [PubMed]
- Doss, M. INWORKS study does not provide evidence for increase in solid cancers from protracted exposure to low doses of ionizing radiation. Lancet Haematol. 2015, 2, e404–e405. [Google Scholar] [CrossRef]
- Scott, B.R. A critique of recent epidemiologic studies of cancer mortality among nuclear workers. Dose-Response 2018. [Google Scholar] [CrossRef] [PubMed]
- Langner, I.; Blettner, M.; Gundestrup, M.; Storm, H.; Aspholm, R.; Auvinen, A.; Pukkala, E.; Hammer, G.P.; Zeeb, H.; Hrafnkelsson, J.; et al. Cosmic radiation and cancer mortality among airline pilots: Results from a European cohort study (ESCAPE). Radiat. Environ. Biophys. 2004, 42, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Berrington, A.; Darby, S.C.; Weiss, H.A.; Doll, R. 100 years of observation on British radiologists: Mortality from cancer and other causes 1897–1997. Br. J. Radiol. 2001, 74, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Linet, M.S.; Kitahara, C.M.; Ntowe, E.; Kleinerman, R.A.; Gilbert, E.S.; Naito, N.; Lipner, R.S.; Miller, D.L.; Berrington de Gonzalez, A.; Multi-Specialty Occupational Health Group. Mortality in U.S. physicians likely to perform fluoroscopy-guided interventional procedures compared with psychiatrists, 1979 to 2008. Radiology 2017, 284, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.S.; Salotti, J.A.; Little, M.P.; McHugh, K.; Lee, C.; Kim, K.P.; Howe, N.L.; Ronckers, C.M.; Rajaraman, P.; Craft, A.W.; et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet 2012, 380, 499–505. [Google Scholar] [CrossRef]
- Mathews, J.D.; Forsythe, A.V.; Zoe Brady, Z.; Butler, M.W.; Goergen, S.K.; Byrnes, G.B.; Graham, G.G.; Wallace, A.B.; Anderson, P.R.; Guiver, T.A.; et al. Cancer risk in 680 000 people exposed to computed tomography scans in childhood or adolescence: Data linkage study of 11 million Australians. BMJ 2013, 346, f2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Journy, N.; Rehel, J.L.; Le Pointe, H.D.; Lee, C.; Brisse, H.; Chateil, J.F.; Caer-Lorho, S.; Laurier, D.; Bernier, M.O. Are the studies on cancer risk from CT scans biased by indication? Elements of answer from a large-scale cohort study in France. Br. J. Cancer 2015, 112, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.A.; Sacks, B.; Pennington, C.W.; Welsh, J.S. Dose optimization to minimize radiation risk for children undergoing CT and nuclear medicine imaging is misguided and detrimental. J. Nucl. Med. 2017, 58, 865–868. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalez, A.B.; Salotti, J.A.; McHugh, K.; Little, M.P.; Harbron, R.W.; Lee, C.; Ntowe, E.; Braganza, M.Z.; Parker, L.; Rajaraman, P.; et al. Relationship between paediatric CT scans and subsequent risk of leukaemia and brain tumours: Assessment of the impact of underlying conditions. Br. J. Cancer 2016, 114, 388–394. [Google Scholar] [CrossRef] [PubMed]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Cuttler, J.M.; Moore, E.R.; Hosfeld, V.D.; Nadolski, D.L. Second update on a patient with Alzheimer disease treated by CT scans. Dose-Response 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P. The exposome: From concept to utility. Int. J. Epidemiol. 2012, 41, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, S.M.; Smith, M.T. Environment and disease risks. Science 2010, 330, 460–461. [Google Scholar] [CrossRef] [PubMed]



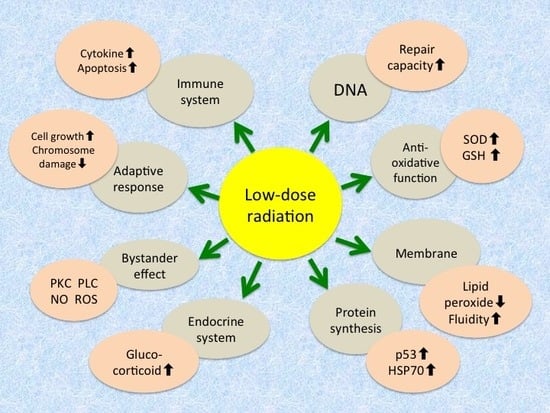
| Level | Mechanism/Phenomenon |
|---|---|
| Molecular | Increase in antioxidative function - Induction of antioxidant enzymes like superoxide dismutase and catalase - Increase in glutathione and thioredoxin levels |
| Increase in repair capacity - Increase in DNA repair enzymes - Activation of poly(ADP-ribose) polymerase | |
| Induction of protein synthesis - Expression of tumor suppressor gene p53 - Induction of stress proteins like HSP70 | |
| Intensification of cellular membrane structure and function - Decrease in lipid peroxides - Increase in membrane fluidity - Increase in Na+/K+-ATPase activity | |
| Cellular | Induction of adaptive response - Increase in cellular proliferation - Decrease in chromosome aberration |
| Increase in immunological activity - Increase in blast transformation and cytokine production - Elimination of damaged cells by apoptosis - Apoptosis of lymphocytes | |
| Radioprotective bystander effects - Transmission of signaling molecules through gap junction - Interaction of factors secreted from irradiated cells - Association of protein kinase C, phospholipase C, nitric oxide, reactive oxygen species, etc. | |
| Endocrine response - Release of glucocorticoids |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibamoto, Y.; Nakamura, H. Overview of Biological, Epidemiological, and Clinical Evidence of Radiation Hormesis. Int. J. Mol. Sci. 2018, 19, 2387. https://doi.org/10.3390/ijms19082387
Shibamoto Y, Nakamura H. Overview of Biological, Epidemiological, and Clinical Evidence of Radiation Hormesis. International Journal of Molecular Sciences. 2018; 19(8):2387. https://doi.org/10.3390/ijms19082387
Chicago/Turabian StyleShibamoto, Yuta, and Hironobu Nakamura. 2018. "Overview of Biological, Epidemiological, and Clinical Evidence of Radiation Hormesis" International Journal of Molecular Sciences 19, no. 8: 2387. https://doi.org/10.3390/ijms19082387
APA StyleShibamoto, Y., & Nakamura, H. (2018). Overview of Biological, Epidemiological, and Clinical Evidence of Radiation Hormesis. International Journal of Molecular Sciences, 19(8), 2387. https://doi.org/10.3390/ijms19082387