Characterizing the Fused TvG6PD::6PGL Protein from the Protozoan Trichomonas vaginalis, and Effects of the NADP+ Molecule on Enzyme Stability
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cloning of TvG6PD::6PGL
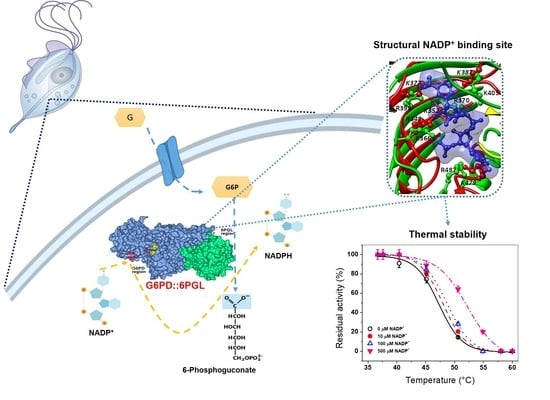
2.2. Homology Modeling of TvG6PD::6PGL
2.3. Expression and Purification of the TvG6PD::6PGL Protein
2.4. Functional Analysis of the TvG6PD::6PGL Protein
2.4.1. Native Status of the TvG6PD::6PGL Protein
2.4.2. Effect of Temperature and pH on TvG6PD::6PGL Activity
2.4.3. Kinetic Characterization of the TvG6PD::6PGL Enzyme
2.5. Evaluation of the Stability of the TvG6PD::6PGL Protein by a NADP+ Molecule
2.5.1. Thermal Inactivation Analysis
2.5.2. Analysis of the Thermal Stability of the TvG6PD::6PGL Protein
2.5.3. Circular Dichroism (CD) of the TvG6PD::6PGL Protein
2.5.4. Susceptibility of the TvG6PD::6PGL Protein to Trypsin Digestion
2.5.5. Susceptibility of TvG6PD::6PGL to Gdn-HCl
2.6. Structural and Spectroscopic Characterization
Determination of the Kd value of Tv G6PD::6PGL from T. vaginalis
3. Materials and Methods
3.1. Culture, RNA Extraction, and cDNA
3.2. Amplification and Cloning of the g6pd::6pgl Gene
3.3. Bioinformatic and Alignment
3.4. Homology Modeling and Comparison of TvG6PD::6PGL
3.5. Expression and Purification
3.6. Functional Analysis of TvG6PD::6PGL Protein
3.6.1. Native Status of the Fused TvG6PD::6PGL Protein
3.6.2. Effect of Temperature and pH on TvG6PD::6PGL Activity
3.6.3. Kinetic Characterization of the TvG6PD::6PGL Enzyme
3.7. Evaluation of the Stability of the TvG6PD::6PGL Protein by the NADP+ Molecule
3.7.1. Thermal Inactivation Analysis
3.7.2. Circular dichroism (CD) of TvG6PD::6PGL protein
3.7.3. Analysis of the Thermal Stability of the TvG6PD::6PGL Protein
3.7.4. Susceptibility of the TvG6PD::6PGL Protein to Trypsin Digestion
3.7.5. Susceptibility of the TvG6PD::6PGL Protein to Guanidine Hydrochloride (Gdn-HCl)
3.8. Structural and Spectroscopic Characterization
Determination of the Ligand Dissociation Constant (Kd) of Structural NADP+
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leitsch, D. Recent advances in the Trichomonas Field. F1000Research 2016, 5, F1000 Faculty Rev-162. [Google Scholar] [CrossRef] [Green Version]
- Johnston, V.J.; Mabey, D.C. Global epidemiology and control of Trichomonas vaginalis. Curr. Opin. Infect. Dis. 2008, 21, 56–64. [Google Scholar] [CrossRef]
- Kusdian, G.; Gould, S.B. The biology of Trichomonas vaginalis in the light of urogenital tract infection. Mol. Biochem. Parasitol. 2014, 198, 92–99. [Google Scholar] [CrossRef]
- Hirt, R.P.; Sherrard, J. Trichomonas vaginalis origins, molecular pathobiology and clinical considerations. Curr. Opin. Infect. Dis. 2015, 28, 72–79. [Google Scholar] [CrossRef] [PubMed]
- McLaren, L.C.; Davis, L.E.; Healy, G.R.; James, C.G. Isolation of Trichomonas vaginalis from the respiratory tract of infants with respiratory disease. Pediatrics 1983, 71, 888–890. [Google Scholar] [PubMed]
- Maritz, J.M.; Land, K.M.; Carlton, J.M.; Hirt, R.P. What is the importance of zoonotic trichomonads for human health? Trends Parasitol. 2014, 30, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Huppert, J.S. Trichomoniasis in teens: An update. Curr. Opin. Obstet. Gynecol. 2009, 1, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Silver, B.J.; Guy, R.J.; Kaldor, J.M.; Jamil, M.S.; Rumbold, A.R. Trichomonas vaginalis as a cause of perinatal morbidity: A systematic review and meta-analysis. Sex. Transm. Dis. 2014, 41, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Masha, S.C.; Cools, P.; Sanders, E.J.; Vaneechoutte, M.; Crucitti, T. Trichomonas vaginalis and HIV infection acquisition: A systematic review and meta-analysis. Sex. Transm. Infect. 2019, 95, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Nanda, N.; Michel, R.G.; Kurdgelashvili, G.; Wendel, K.A. Trichomoniasis and its treatment. Expert Rev. Anti Infect. Ther. 2006, 4, 125–135. [Google Scholar] [CrossRef]
- Sutcliffe, S.; Giovannucci, E.; Alderete, J.F.; Chang, T.H.; Gaydos, C.A.; Zenilman, J.M.; De Marzo, A.M.; Willett, W.C.; Platz, E.A. Plasma antibodies against Trichomonas vaginalis and subsequent risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. 2006, 15, 939–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harp, D.F.; Chowdhury, I. Trichomoniasis: Evaluation to execution. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 157, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sood, S.; Arti, K. An update on Trichomonas vaginalis. Indian J. Sex. Transm. Dis. AIDS 2008, 29, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Schneider, R.E.; Brown, M.T.; Shiflett, A.M.; Dyall, S.D.; Hayes, R.D.; Xie, Y.; Loo, J.A.; Johnson, P.J. The Trichomonas vaginalis hydrogenosome proteome is highly reduced relative to mitochondria, yet complex compared with mitosomes. Int. J. Parasitol. 2011, 41, 1421–1434. [Google Scholar] [CrossRef] [Green Version]
- Fichorova, R.N.; Trifonova, R.T.; Gilbert, R.O.; Costello, C.E.; Hayes, G.R.; Lucas, J.J.; Singh, B.N. Trichomonas vaginalis lipophosphoglycan triggers a selective upregulation of cytokines by human female reproductive tract epithelial cells. Infect. Immun. 2006, 74, 5773–5779. [Google Scholar] [CrossRef] [Green Version]
- Lamien Meda, A.; Leitsch, D. Identification of the NADH-oxidase gene in Trichomonas vaginalis. Parasitol. Res. 2020, 119, 683–686. [Google Scholar] [CrossRef] [Green Version]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelin, M.M.C.; Campbell, K.; Cheung, E.; Viridiana, O.S.; Nana, G.; Antje, K.; Mohammad, T.; et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. Camb. Philos. Soc. 2015, 90, 927–963. [Google Scholar] [CrossRef] [Green Version]
- Alencar, N.; Sola, I.; Linares, M.; Juárez-Jiménez, J.; Pont, C.; Viayna, A.; Vílchez, D.; Sampedro, C.; Abad, P.; Pérez-Benavente, S.; et al. First homology model of Plasmodium falciparum glucose-6-phosphate dehydrogenase: Discovery of selective substrate analog-based inhibitors as novel antimalarial agents. Eur. J. Med. Chem. 2018, 146, 108–122. [Google Scholar] [CrossRef]
- Stover, N.A.; Dixon, T.A.; Cavalcanti, A.R. Multiple independent fusions of glucose-6-phosphate dehydrogenase with enzymes in the pentose phosphate pathway. PLoS ONE 2011, 6, e22269. [Google Scholar] [CrossRef] [Green Version]
- Carlton, J.M.; Hirt, R.P.; Silva, J.C.; Delcher, A.L.; Schatz, M.; Zhao, Q.; Wortman, J.R.; Bidwell, S.L.; Alsmark, U.C.; Besteiro, S.; et al. Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science 2007, 315, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Clarke, J.L.; Scopes, D.A.; Sodeinde, O.; Mason, P.J. Glucose-6-phosphate dehydrogenase-6-phosphogluconolactonase. A novel bifunctional enzyme in malaria parasites. Eur. J. Biochem. 2001, 268, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Jortzik, E.; Mailu, B.M.; Preuss, J.; Fischer, M.; Bode, L.; Rahlfs, S.; Becker, K. Glucose-6-phosphate dehydrogenase-6-phosphogluconolactonase: A unique bifunctional enzyme from Plasmodium falciparum. Biochem. J. 2011, 436, 641–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Luna, L.; Serrano-Posada, H.; González-Valdez, A.; Ortega-Cuellar, D.; Vanoye-Carlo, A.; Hernández-Ochoa, B.; Sierra-Palacios, E.; Rufino-González, Y.; Castillo-Rodríguez, R.A.; Pérez de la Cruz, V.; et al. Biochemical Characterization and Structural Modeling of Fused Glucose-6-Phosphate Dehydrogenase-Phosphogluconolactonase from Giardia lamblia. Int. J. Mol. Sci. 2018, 19, 2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, H.G.; McArthur, A.G.; Gillin, F.D.; Aley, S.B.; Adam, R.D.; Olsen, G.J.; Best, A.A.; Cande, W.Z.; Chen, F.; Cipriano, M.J. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science 2007, 317, 1921–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuda, K.; Nakamura, T.; Sugita, M.; Shimizu, T.; Shikanai, T. A pentatricopeptide repeat protein is a site recognition factor in chloroplast RNA editing. J. Biol. Chem. 2006, 281, 37661–37667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotaka, M.; Gover, S.; Vandeputte-Rutten, L.; Au, S.W.; Lam, V.M.; Adams, M.J. Structural studies of glucose-6-phosphate and NADP(+) binding to human glucose-6-phosphate dehydrogenase. Acta Crystallogr. 2005, 61, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Rowland, P.; Basak, A.K.; Gover, S.; Levy, H.R.; Adams, M.J. The three-dimensional structure of glucose 6-phosphate dehydrogenase from Leuconostoc mesenteroides refined at 2.0 Å resolution. Structure 1994, 2, 1073–1087. [Google Scholar] [CrossRef] [Green Version]
- Camardella, L.; Caruso, C.; Rutigliano, B.; Romano, M.; Di Prisco, G.; Descalzi-Cancedda, F. Human erythrocyte glucose-6-phosphate dehydrogenase. Identification of a reactive lysyl residue labelled with pyridoxal 5′-phosphate. Eur. J. Biochem. 1988, 171, 485–489. [Google Scholar] [CrossRef]
- Bautista, J.M.; Mason, P.J.; Luzzatto, L. Human glucose-6-phosphate dehydrogenase. Lysine 205 is dispensable for substrate binding but essential for catalysis. FEBS Lett. 1995, 366, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modelling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [Green Version]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Acero-Navarro, K.E.; Jiménez-Ramírez, M.; Villalobos, M.A.; Vargas-Martínez, R.; Perales-Vela, H.V.; Velasco-García, R. Cloning, overexpression, and purification of glucose-6-phosphate dehydrogenase of Pseudomonas aeruginosa. Protein Expr. Purif. 2018, 142, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Suthar, M.K.; Doharey, P.K.; Gupta, S.; Yadav, S.; Chauhan, P.M.; Saxena, J.K. Molecular cloning and characterization of glucose-6-phosphate dehydrogenase from Brugia malayi. Parasitology 2013, 140, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.; Schlichting, B.; Schonheit, P. Glucose-6-phosphate dehydrogenase from the hyperthermophilic bacterium Thermotoga maritima: Expression of the g6pd gene and characterization of an extremely thermophilic enzyme. FEMS Microbiol. Lett. 2002, 216, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Ghazy, A.M.; Salem, A.M.H.; Ghazy, M.A.; Abdel-Monsef, M.M. Purification and characterization of glucose-6-phosphate dehydrogenase from camel liver. Enzym. Res. 2014, 2014, 714054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, M.A.; Ghazy, A.H.; Salem, A.M.; Ghazy, M.A.; Abdel-Monsef, M.M. Biochemical characterization of buffalo liver glucose-6-phosphate dehydrogenase isoforms. Protein J. 2015, 34, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; Lam, V.M.; Engel, P.C. Marked decrease in specific activity contributes to disease phenotype in two human glucose-6-phosphate dehydrogenase mutants, G6PD Union and G6PD Andalus. Hum. Mutat. 2005, 26, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Özer, N.; Bilgi, C.; Ögüsa, I.H. Dog liver glucose-6-phosphate dehydrogenase: Purification and kinetic properties. Int. J. Biochem. Cell. Biol. 2002, 34, 253–262. [Google Scholar] [CrossRef]
- Rendón, J.L.; del Arenal, I.P.; Guevara-Flores, A.; Mendoza-Hernández, G.; Pardo, J.P. Glucose 6-phosphate dehydrogenase from larval Taenia crassiceps (cysticerci): Purification and properties. Parasitol. Res. 2008, 102, 1351–1357. [Google Scholar] [CrossRef]
- Ortiz, C.; Moraca, F.; Medeiros, A.; Botta, M.; Hamilton, N.; Comini, M.A. Binding mode and selectivity of steroids towards glucose-6-phosphate dehydrogenase from the pathogen Trypanosoma cruzi. Molecules 2016, 21, 368. [Google Scholar] [CrossRef] [Green Version]
- Haeussler, K.; Berneburg, I.; Jortzik, E.; Hahn, J.; Rahbari, M.; Schulz, N.; Kaufmann, D.E. Glucose 6-phosphate dehydrogenase 6-phosphogluconolactonase: Characterization of the Plasmodium vivax enzyme and inhibitor studies. Malar. J. 2019, 18, 22. [Google Scholar] [CrossRef]
- Gómez-Manzo, S.; Terrón-Hernández, J.; De la Mora-De la Mora, I.; González-Valdez, A.; Marcial-Quino, J.; García-Torres, I.; Vanoye-Carlo, A.; López-Velázquez, G.; Hernández-Alcántara, G.; Oria-Hernández, J.; et al. The Stability of G6PD Is Affected by Mutations with Different Clinical Phenotypes. Int. J. Mol. Sci. 2014, 15, 21179–21201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffieux, F.; Van Roy, J.; Michels, P.A.; Opperdoes, F.R. Molecular characterization of the first two enzymes of the pentose-phosphate pathway of Trypanosoma brucei. Glucose-6-phosphate dehydrogenase and 6-phosphogluconolactonase. J. Biol. Chem. 2000, 275, 27559–27565. [Google Scholar] [PubMed] [Green Version]
- Ramírez-Nava, E.J.; Ortega-Cuellar, D.; González-Valdez, A.; Castillo-Rodríguez, R.A.; Ponce-Soto, G.Y.; Hernández-Ochoa, B.; Cárdenas-Rodríguez, N.; Martínez-Rosas, V.; Morales-Luna, L.; Serrano-Posada, H.; et al. Molecular Cloning and Exploration of the Biochemical and Functional Analysis of Recombinant Glucose-6-Phosphate Dehydrogenase from Gluconoacetobacter diazotrophicus PAL5. Int. J. Mol. Sci. 2019, 20, 5279. [Google Scholar] [CrossRef]
- Morales-Luna, L.; González-Valdez, A.; Sixto-López, Y.; Correa-Basurto, J.; Hernández-Ochoa, B.; Cárdenas-Rodríguez, N.; Castillo-Rodríguez, N.; Castillo-Rodríguez, R.A.; Ortega-Cuellar, D.; Arreguin-Espinosa, R.; et al. Identification of the NADP+ Structural Binding Site and Coenzyme Effect on the Fused G6PD::6PGL Protein from Giardia lamblia. Biomolecules 2018, 10, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez-Nava, E.J.; Ortega-Cuellar, D.; Serrano-Posada, H.; González-Valdez, A.; Vanoye-Carlo, A.; Hernández-Ochoa, B.; Sierra-Palacios, E.; Hernández-Pineda, J.; Rodríguez-Bustamante, E.; Arreguin-Espinosa, R.; et al. Biochemical Analysis of Two Single Mutants that Give Rise to a Polymorphic G6PD A-Double Mutant. Int. J. Mol. Sci. 2017, 18, 2244. [Google Scholar] [CrossRef] [Green Version]
- Cortés-Morales, Y.Y.; Vanoye-Carlo, A.; Castillo-Rodríguez, R.A.; Serrano-Posada, H.; González-Valdez, A.; Ortega-Cuellar, D.; Hernández-Ochoa, B.; Moreno-Vargas, L.M.; Prada-Gracia, D.; Sierra-Palacios, E.; et al. Cloning and biochemical characterization of three glucose 6 phosphate dehydrogenase mutants presents in the Mexican population. Int. J. Biol. Macromol. 2018, 119, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Au, S.W.N.; Gover, S.; Lam, V.M.S.; Adams, M. Human glucose-6-phosphate dehydrogenase: The crystal structure reveals a structural NADP+ molecule and provides insights into enzyme deficiency. Structure 2000, 8, 293–303. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.T.; Chan, T.F.; Lam, V.; Engel, P. What is the role of the second “structural” NADP-binding site in human glucose-6-phosphate dehydrogenase? Protein Sci. 2008, 17, 1403–1411. [Google Scholar] [CrossRef] [Green Version]
- Preuss, J.; Hedrick, M.; Sergienko, E.; Pinkerton, A.; Mangravita-Novo, A.; Smith, L.; Marx, C.; Fischer, E.; Jortzik, E.; Rahlfs, S.; et al. High-throughput screening for small-molecule inhibitors of Plasmodium falciparum glucose-6-phosphate dehydrogenase 6-phosphogluconolactonase. J. Biomol. Screen. 2012, 17, 738–751. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.G.; Diamond, L.S. Methods for cultivation of luminal parasitic protists of clinical importance. Clin. Microbiol. Rev. 2002, 15, 329–341. [Google Scholar] [CrossRef] [Green Version]
- Fu, A.Y.; Spence, C.; Scherer, A.; Arnold, F.H.; Quake, S.R. A microfabricated fluorescence-activated cell sorter. Nat. Biotechnol. 1999, 17, 1109–1111. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Krieger, E.; Joo, K.; Lee, J.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins 2009, 77, 114–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, V.B.; Arendall, W.B., 3rd; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 2010, 66(Pt. 1), 12–21. [Google Scholar] [CrossRef] [Green Version]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Beer, T.A.; Berka, K.; Thornton, J.M.; Laskowski, R.A. PDBsum additions. Nucleic Acids Res. 2014, 42, D292–D296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNicholas, S.; Potterton, E.; Wilson, K.S.; Noble, M.E. Presenting your structures: The CCP4mg molecular-graphics software. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 386–394. [Google Scholar] [CrossRef] [Green Version]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Gómez-Manzo, S.; Marcial-Quino, J.; Vanoye-Carlo, A.; Enríquez-Flores, S.; De la Mora-De la Mora, I.; González-Valdez, A.; García-Torres, I.; Martínez-Rosas, V.; Sierra-Palacios, E.; Lazcano-Pérez, F.; et al. Mutations of Glucose-6-Phosphate Dehydrogenase Durham, Santa-Maria and A+ Variants Are Associated with Loss Functional and Structural Stability of the Protein. Int. J. Mol. Sci. 2015, 16, 28657–28668. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Manzo, S.; Marcial-Quino, J.; Vanoye-Carlo, A.; Serrano-Posada, H.; Ortega-Cuellar, D.; González-Valdez, A.; Castillo-Rodríguez, R.A.; Hernández-Ochoa, B.; Sierra-Palacios, E.; Rodríguez-Bustamante, E.; et al. Glucose-6-Phosphate Dehydrogenase: Update and Analysis of New Mutations around the World. Int. J. Mol. Sci. 2016, 17, 2069. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Manzo, S.; Terrón-Hernández, J.; de la Mora-de la Mora, I.; García-Torres, I.; López-Velázquez, G.; Reyes-Vivas, H.; Oria-Hernández, J. Cloning, expression, purification and characterization of his-tagged human glucose-6-phosphate dehydrogenase: A simplified method for protein yield. Protein J. 2013, 32, 585–592. [Google Scholar] [CrossRef] [PubMed]









| G6PD from Organism | Km G6P (mM) | Km NADP+ (mM) | Vmax (µmol·min−1·mg -1) | kcat (s−1) | Reference |
|---|---|---|---|---|---|
| T. vaginalis | 0.21 | 0.027 | 108.6 | 147 | This study |
| G. lamblia | 0.018 | 0.013 | 11.5 | 31.8 | [23] |
| P. falciparum | 0.019 | 0.006 | 5.2 | 8.6 | [22] |
| P. vivax | 0.080 | 0.014 | 5.6 | 6.7 | [41] |
| T. cruzi | 0.077 | 0.0016 | NR | 53.6 | [40] |
| T. brucei | 0.138 | 0.035 | 740.1 | NR | [43] |
| Homo sapiens | 0.038 | 0.007 | 160.1 | 230.3 | [42] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Luna, L.; Hernández-Ochoa, B.; Ramírez-Nava, E.J.; Martínez-Rosas, V.; Ortiz-Ramírez, P.; Fernández-Rosario, F.; González-Valdez, A.; Cárdenas-Rodríguez, N.; Serrano-Posada, H.; Centeno-Leija, S.; et al. Characterizing the Fused TvG6PD::6PGL Protein from the Protozoan Trichomonas vaginalis, and Effects of the NADP+ Molecule on Enzyme Stability. Int. J. Mol. Sci. 2020, 21, 4831. https://doi.org/10.3390/ijms21144831
Morales-Luna L, Hernández-Ochoa B, Ramírez-Nava EJ, Martínez-Rosas V, Ortiz-Ramírez P, Fernández-Rosario F, González-Valdez A, Cárdenas-Rodríguez N, Serrano-Posada H, Centeno-Leija S, et al. Characterizing the Fused TvG6PD::6PGL Protein from the Protozoan Trichomonas vaginalis, and Effects of the NADP+ Molecule on Enzyme Stability. International Journal of Molecular Sciences. 2020; 21(14):4831. https://doi.org/10.3390/ijms21144831
Chicago/Turabian StyleMorales-Luna, Laura, Beatriz Hernández-Ochoa, Edson Jiovany Ramírez-Nava, Víctor Martínez-Rosas, Paulina Ortiz-Ramírez, Fabiola Fernández-Rosario, Abigail González-Valdez, Noemí Cárdenas-Rodríguez, Hugo Serrano-Posada, Sara Centeno-Leija, and et al. 2020. "Characterizing the Fused TvG6PD::6PGL Protein from the Protozoan Trichomonas vaginalis, and Effects of the NADP+ Molecule on Enzyme Stability" International Journal of Molecular Sciences 21, no. 14: 4831. https://doi.org/10.3390/ijms21144831
APA StyleMorales-Luna, L., Hernández-Ochoa, B., Ramírez-Nava, E. J., Martínez-Rosas, V., Ortiz-Ramírez, P., Fernández-Rosario, F., González-Valdez, A., Cárdenas-Rodríguez, N., Serrano-Posada, H., Centeno-Leija, S., Arreguin-Espinosa, R., Cuevas-Cruz, M., Ortega-Cuellar, D., Pérez de la Cruz, V., Rocha-Ramírez, L. M., Sierra-Palacios, E., Castillo-Rodríguez, R. A., Vega-García, V., Rufino-González, Y., ... Gómez-Manzo, S. (2020). Characterizing the Fused TvG6PD::6PGL Protein from the Protozoan Trichomonas vaginalis, and Effects of the NADP+ Molecule on Enzyme Stability. International Journal of Molecular Sciences, 21(14), 4831. https://doi.org/10.3390/ijms21144831