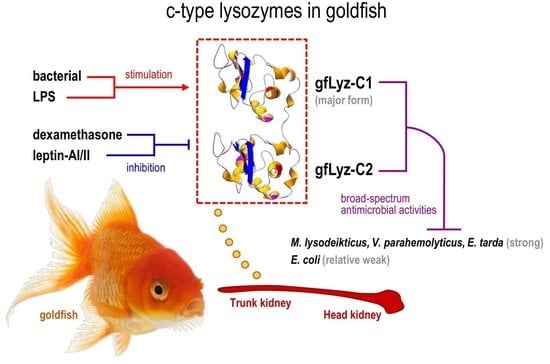
Two Distinct C-Type Lysozymes in Goldfish: Molecular Characterization, Antimicrobial Potential, and Transcriptional Regulation in Response to Opposing Effects of Bacteria/Lipopolysaccharide and Dexamethasone/Leptin
Abstract
:1. Introduction
2. Results
2.1. Molecular Cloning and Bioinformatics Analysis of Two Goldfish C-Type Lysozymes
2.2. Phylogenetic Analysis and Sequence Alignment of Lysozymes in Different Species
2.3. Bacteriolytic and Bactericidal Activities of the Recombinant gfLyz-C1 and gfLyz-C2 Proteins
2.4. Tissue Distribution and Bacterial Induction of gfLyz-C1 and gfLyz-C2 mRNA Expression
2.5. Changes in the Expression of gfLyz-C1 and gfLyz-C2 Transcripts in Goldfish Primary Trunk Kidney Cells in Response to Immune Challenge and Hormonal Treatment
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Molecular Cloning of Two C-Type Lysozyme cDNAs from Goldfish
4.3. Expression and Purification of the Recombinant gfLyz-C1 and gfLyz-C2 Proteins
4.4. Lysoplate Assay and Turbidimetric Assay
4.5. Tissue Distribution of Goldfish C-Type Lysozyme mRNAs
4.6. Immune Challenge of Goldfish In Vivo
4.7. Isolation, Primary Culture, and Static Incubation of Goldfish Trunk Kidney Cells
4.8. Measurement of gfLyz-C1 or gfLyz-C2 mRNA Levels
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jolles, P.; Jolles, J. Whats New in Lysozyme Research—Always a Model System, Today as Yesterday. Mol. Cell. Biochem. 1984, 63, 165–189. [Google Scholar] [PubMed]
- Vocadlo, D.J.; Davies, G.J.; Laine, R.; Withers, S.G. Catalysis by hen egg-white lysozyme proceeds via a covalent intermediate. Nature 2001, 412, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Diamond, G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000, 8, 402–410. [Google Scholar] [CrossRef]
- Callewaert, L.; Michiels, C.W. Lysozymes in the animal kingdom. J. Biosci. 2010, 35, 127–160. [Google Scholar] [CrossRef]
- Bachali, S.; Jager, M.; Hassanin, A.; Schoentgen, F.; Jolles, P.; Fiala-Medioni, A.; Deutsch, J.S. Phylogenetic analysis of invertebrate lysozymes and the evolution of lysozyme function. J. Mol. Evol. 2002, 54, 652–664. [Google Scholar] [CrossRef]
- Irwin, D.M.; Gong, Z.Y. Molecular evolution of vertebrate goose-type lysozyme genes. J. Mol. Evol. 2003, 56, 234–242. [Google Scholar] [CrossRef]
- Jung, A.; Sippel, A.E.; Grez, M.; Schutz, G. Exons encode functional and structural units of chicken lysozyme. Proc. Natl. Acad. Sci. USA 1980, 77, 5759–5763. [Google Scholar] [CrossRef] [Green Version]
- Peters, C.W.B.; Kruse, U.; Pollwein, R.; Grzeschik, K.H.; Sippel, A.E. The Human Lysozyme Gene—Sequence Organization and Chromosomal Localization. Eur. J. Biochem. 1989, 182, 507–516. [Google Scholar] [CrossRef]
- Cross, M.; Mangelsdorf, I.; Wedel, A.; Renkawitz, R. Mouse Lysozyme-M Gene—Isolation, Characterization, and Expression Studies. Proc. Natl. Acad. Sci. USA 1988, 85, 6232–6236. [Google Scholar] [CrossRef] [Green Version]
- Yeh, T.C.; Wilson, A.C.; Irwin, D.M. Evolution of rodent lysozymes: Isolation and sequence of the rat lysozyme genes. Mol. Phylogenet. Evol. 1993, 2, 65–75. [Google Scholar] [CrossRef]
- Dautigny, A.; Prager, E.M.; Phamdinh, D.; Jolles, J.; Pakdel, F.; Grinde, B.; Jolles, P. Cdna and Amino-Acid-Sequences of Rainbow-Trout (Oncorhynchus-Mykiss) Lysozymes and Their Implications for the Evolution of Lysozyme and Lactalbumin. J. Mol. Evol. 1991, 32, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Hikima, J.; Hirono, I.; Aoki, T. Characterization and expression of c-type lysozyme cDNA from Japanese flounder (Paralichthys olivaceus). Mol. Mar. Biol. Biotech. 1997, 6, 339–344. [Google Scholar]
- Hikima, J.; Hirono, I.; Aoki, T. Molecular cloning and novel repeated sequences of a C-type lysozyme gene in Japanese flounder (Paralichthys olivaceus). Mar. Biotechnol. 2000, 2, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Cantizano, R.M.; Infante, C.; Martin-Antonio, B.; Ponce, M.; Hachero, I.; Navas, J.I.; Manchado, M. Molecular characterization, phylogeny, and expression of c-type and g-type lysozymes in brill (Scophthalmus rhombus). Fish Shellfish Immunol. 2008, 25, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Trujillo, M.A.; Porta, J.; Manchado, M.; Borrego, J.J.; Alvarez, M.C.; Bejar, J. c-Lysozyme from Senegalese sole (Solea senegalensis): cDNA cloning and expression pattern. Fish Shellfish Immunol. 2008, 25, 697–700. [Google Scholar] [CrossRef]
- Liu, F.; Wen, Z.L. Cloning and expression pattern of the lysozyme C gene in zebrafish. Mech. Dev. 2002, 113, 69–72. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, L.; Tian, Y.; Tan, A.; Bai, J.; Li, S. Identification and expression analysis of the g-type and c-type lysozymes in grass carp Ctenopharyngodon idellus. Dev. Comp. Immunol. 2010, 34, 501–509. [Google Scholar] [CrossRef]
- Gao, F.Y.; Qu, L.; Yu, S.G.; Ye, X.; Tian, Y.Y.; Zhang, L.L.; Bai, J.J.; Lu, M.X. Identification and expression analysis of three c-type lysozymes in Oreochromis aureus. Fish Shellfish Immunol. 2012, 32, 779–788. [Google Scholar] [CrossRef]
- Wei, S.; Huang, Y.; Cai, J.; Huang, X.; Fu, J.; Qin, Q. Molecular cloning and characterization of c-type lysozyme gene in orange-spotted grouper, Epinephelus coioides. Fish Shellfish Immunol. 2012, 33, 186–196. [Google Scholar] [CrossRef]
- Pridgeon, J.W.; Klesius, P.H.; Dominowski, P.J.; Yancey, R.J.; Kievit, M.S. Chicken-type lysozyme in channel catfish: Expression analysis, lysozyme activity, and efficacy as immunostimulant against Aeromonas hydrophila infection. Fish Shellfish Immunol. 2013, 35, 680–688. [Google Scholar] [CrossRef]
- Wang, M.J.; Zhao, X.L.; Kong, X.H.; Wang, L.; Jiao, D.; Zhang, H.X. Molecular characterization and expressing analysis of the c-type and g-type lysozymes in Qihe crucian carp Carassius auratus. Fish Shellfish Immunol. 2016, 52, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Xu, Q.Q.; Boscari, E.; Du, H.; Qi, Z.T.; Li, Y.S.; Huang, J.; Di, J.; Yue, H.M.; Li, C.J.; et al. Characterization and expression analysis of g- and c-type lysozymes in Dabry’s sturgeon (Acipenser dabryanus). Fish Shellfish Immunol. 2018, 76, 260–265. [Google Scholar] [CrossRef]
- Minagawa, S.; Hikima, J.; Hirono, I.; Aoki, T.; Mori, H. Expression of Japanese flounder c-type lysozyme cDNA in insect cells. Dev. Comp. Immunol. 2001, 25, 439–445. [Google Scholar] [CrossRef]
- Chen, T.; Wong, M.K.H.; Chan, B.C.B.; Wong, A.O.L. Mechanisms for Temperature Modulation of Feeding in Goldfish and Implications on Seasonal Changes in Feeding Behavior and Food Intake. Front. Endocrinol. 2019, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Omori, Y.; Koren, S.; Shirokiya, T.; Kuroda, T.; Miyamoto, A.; Wada, H.; Fujiyama, A.; Toyoda, A.; Zhang, S.; et al. De novo assembly of the goldfish (Carassius auratus) genome and the evolution of genes after whole-genome duplication. Sci. Adv. 2019, 5, eaav0547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omori, Y.; Kon, T. Goldfish: An old and new model system to study vertebrate development, evolution and human disease. J. Biochem. 2019, 165, 209–218. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, X.; Wang, X.; Li, J.; Liu, G.; Kuang, Y.; Xu, J.; Zheng, X.; Ren, L.; Wang, G.; et al. Genome sequence and genetic diversity of the common carp, Cyprinus carpio. Nat. Genet. 2014, 46, 1212–1219. [Google Scholar] [CrossRef] [Green Version]
- Kaizu, A.; Fagutao, F.F.; Kondo, H.; Aoki, T.; Hirono, I. Functional Analysis of C-type Lysozyme in Penaeid Shrimp. J. Biol. Chem. 2011, 286, 44344–44349. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Ren, C.; Wang, Y.; Luo, P.; Jiang, X.; Huang, W.; Chen, C.; Hu, C. Molecular cloning, inducible expression and antibacterial analysis of a novel i-type lysozyme (lyz-i2) in Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2016, 54, 197–203. [Google Scholar] [CrossRef]
- Beckert, A.; Wiesner, J.; Baumann, A.; Poppel, A.K.; Vogel, H.; Vilcinskas, A. Two c-type lysozymes boost the innate immune system of the invasive ladybird Harmonia axyridis. Dev. Comp. Immunol. 2015, 49, 303–312. [Google Scholar] [CrossRef]
- Yang, D.; Wang, Q.; Cao, R.; Chen, L.; Liu, Y.; Cong, M.; Wu, H.; Li, F.; Ji, C.; Zhao, J. Molecular characterization, expression and antimicrobial activities of two c-type lysozymes from manila clam Venerupis philippinarum. Dev. Comp. Immunol. 2017, 73, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Wild, P.; Gabrieli, A.; Schraner, E.M.; Pellegrini, A.; Thomas, U.; Frederik, P.M.; Stuart, M.C.; Von Fellenberg, R. Reevaluation of the effect of lysoyzme on Escherichia coli employing ultrarapid freezing followed by cryoelectronmicroscopy or freeze substitution. Microsc. Res. Tech. 1997, 39, 297–304. [Google Scholar] [CrossRef]
- Willett, C.E.; Cortes, A.; Zuasti, A.; Zapata, A.G. Early hematopoiesis and developing lymphoid organs in the zebrafish. Dev. Dynam. 1999, 214, 323–336. [Google Scholar] [CrossRef]
- Faust, N.; Varas, F.; Kelly, L.M.; Heck, S.; Graf, T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood 2000, 96, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Shepard, J.L.; Zon, L.I. Developmental derivation of embryonic and adult macrophages. Curr. Opin. Hematol. 2000, 7, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ren, C.H.; Li, W.H.; Jiang, X.; Xia, J.J.; Wong, N.K.; Hu, C.Q. Calmodulin of the tropical sea cucumber: Gene structure, inducible expression and contribution to nitric oxide production and pathogen clearance during immune response. Fish Shellfish Immun. 2015, 45, 231–238. [Google Scholar] [CrossRef]
- Kagawa, N.; Mugiya, Y. Exposure of Goldfish (Carassius auratus) to Bluegills (Lepomis macrochirus) Enhances Expression of Stress Protein 70 mRNA in the Brains and Increases Plasma Cortisol Levels. Zool. Sci. 2000, 17, 1061–1066. [Google Scholar] [CrossRef]
- Kagawa, N.; Mugiya, Y. Brain HSP70 mRNA expression is linked with plasma cortisol levels in goldfish (Carassius auratus) exposed to a potential predator. Zool. Sci. 2002, 19, 735–740. [Google Scholar] [CrossRef] [Green Version]
- Bernier, N.J.; Bedard, N.; Peter, R.E. Effects of cortisol on food intake, growth, and forebrain neuropeptide Y and corticotropin-releasing factor gene expression in goldfish. Gen. Comp. Endocrinol. 2004, 135, 230–240. [Google Scholar] [CrossRef]
- Sanchez-Bretano, A.; Callejo, M.; Montero, M.; Alonso-Gomez, A.L.; Delgado, M.J.; Isorna, E. Performing a hepatic timing signal: Glucocorticoids induce gper1a and gper1b expression and repress gclock1a and gbmal1a in the liver of goldfish. J. Comp. Physiol. B 2016, 186, 73–82. [Google Scholar] [CrossRef]
- Kurokawa, T.; Uji, S.; Suzuki, T. Identification of cDNA coding for a homologue to mammalian leptin from pufferfish, Takifugu rubripes. Peptides 2005, 26, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, S.; Ren, C.; Hu, C.; Tang, D.; Yan, A. Two isoforms of leptin in the White-clouds Mountain minnow (Tanichthys albonubes): Differential regulation by estrogen despite similar response to fasting. Gen. Comp. Endocrinol. 2016, 225, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.F.; Chen, T.; Chen, S.; Ren, C.H.; Hu, C.Q.; Cai, Y.M.; Liu, F.; Tang, D.S. Goldfish Leptin-AI and Leptin-AII: Function and Central Mechanism in Feeding Control. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.F.; Chen, Y.F.; Chen, S.; Li, S.S.; Zhang, Y.; Jia, J.R.; Yu, H.; Liu, L.; Liu, F.; Hu, C.Q.; et al. Leptin Stimulates Prolactin mRNA Expression in the Goldfish Pituitary through a Combination of the PI3K/Akt/mTOR, MKK3/6/p(38)MAPK and MEK1/2/ERK1/2 Signalling Pathways. Int. J. Mol. Sci. 2017, 18, 2781. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, T.; Rao, Y.; Chen, S.; Wang, B.; Chen, R.; Ren, C.; Liu, L.; Yang, Y.; Yu, H.; et al. Suppression of leptin-AI/AII transcripts by insulin in goldfish liver: A fish specific response of leptin under food deprivation. Gen. Comp. Endocrinol. 2019, 283, 113240. [Google Scholar] [CrossRef]
- Yan, A.F.; Chen, T.; Chen, S.; Tang, D.S.; Liu, F.; Jiang, X.; Huang, W.; Ren, C.H.; Hu, C.Q. Signal transduction mechanism for glucagon-induced leptin gene expression in goldfish liver. Int. J. Biol. Sci. 2016, 12, 1544–1554. [Google Scholar] [CrossRef] [Green Version]
- Procaccini, C.; La Rocca, C.; Carbone, F.; De Rosa, V.; Galgani, M.; Matarese, G. Leptin as immune mediator: Interaction between neuroendocrine and immune system. Dev. Comp. Immunol. 2017, 66, 120–129. [Google Scholar] [CrossRef]
- Luo, X.; Chen, T.; Zhong, M.; Jiang, X.; Zhang, L.; Ren, C.; Hu, C. Differential regulation of hepatopancreatic vitellogenin (VTG) gene expression by two putative molt-inhibiting hormones (MIH1/2) in Pacific white shrimp (Litopenaeus vannamei). Peptides 2015, 68, 58–63. [Google Scholar] [CrossRef]
- Jing, H.L.; Gao, L.Y.; Zhang, M.; Wang, N.; Lin, X.M.; Zhang, L.F.; Wu, S.Q. Establishment from the snout and kidney of goldfish, Carassius auratus, of two new cell lines and their susceptibility to infectious pancreatic necrosis virus. Fish Physiol. Biochem. 2016, 42, 303–311. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Rao, Y.; Li, J.; Ren, C.; Tang, D.; Lin, T.; Ji, J.; Chen, R.; Yan, A. Two Distinct C-Type Lysozymes in Goldfish: Molecular Characterization, Antimicrobial Potential, and Transcriptional Regulation in Response to Opposing Effects of Bacteria/Lipopolysaccharide and Dexamethasone/Leptin. Int. J. Mol. Sci. 2020, 21, 501. https://doi.org/10.3390/ijms21020501
Chen T, Rao Y, Li J, Ren C, Tang D, Lin T, Ji J, Chen R, Yan A. Two Distinct C-Type Lysozymes in Goldfish: Molecular Characterization, Antimicrobial Potential, and Transcriptional Regulation in Response to Opposing Effects of Bacteria/Lipopolysaccharide and Dexamethasone/Leptin. International Journal of Molecular Sciences. 2020; 21(2):501. https://doi.org/10.3390/ijms21020501
Chicago/Turabian StyleChen, Ting, Yingzhu Rao, Jiaxi Li, Chunhua Ren, Dongsheng Tang, Tiehao Lin, Jiatai Ji, Rong Chen, and Aifen Yan. 2020. "Two Distinct C-Type Lysozymes in Goldfish: Molecular Characterization, Antimicrobial Potential, and Transcriptional Regulation in Response to Opposing Effects of Bacteria/Lipopolysaccharide and Dexamethasone/Leptin" International Journal of Molecular Sciences 21, no. 2: 501. https://doi.org/10.3390/ijms21020501
APA StyleChen, T., Rao, Y., Li, J., Ren, C., Tang, D., Lin, T., Ji, J., Chen, R., & Yan, A. (2020). Two Distinct C-Type Lysozymes in Goldfish: Molecular Characterization, Antimicrobial Potential, and Transcriptional Regulation in Response to Opposing Effects of Bacteria/Lipopolysaccharide and Dexamethasone/Leptin. International Journal of Molecular Sciences, 21(2), 501. https://doi.org/10.3390/ijms21020501