Evaluation of Thio- and Seleno-Acetamides Bearing Benzenesulfonamide as Inhibitor of Carbonic Anhydrases from Different Pathogenic Bacteria
Abstract
:1. Introduction
2. Results and Discussion
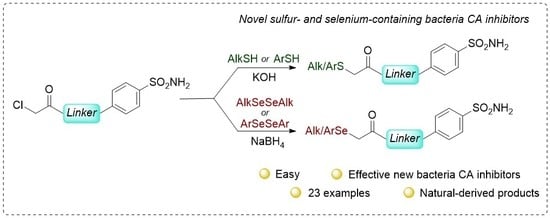
2.1. Chemistry
2.2. Carbonic Anhydrase Inhibition
3. Materials and Methods
3.1. General
3.2. Chemistry
3.3. Carbonic Anhydrase Inhibition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CA | Carbonic Anhydrase |
| CAI | Carbonic Anhydrase Inhibitors |
References
- van Hecke, O.; Wang, K.; Lee, J.J.; Roberts, N.W.; Butler, C.C. Implications of Antibiotic Resistance for Patients’ Recovery From Common Infections in the Community: A Systematic Review and Meta-analysis. Clin. Infect Dis. 2017, 65, 371–382. [Google Scholar] [CrossRef]
- Williamson, D.A.; Carter, G.P.; Howden, B.P. Current and Emerging Topical Antibacterials and Antiseptics: Agents, Action, and Resistance Patterns. Clin. Microbiol. Rev. 2017, 30, 827–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ching, C.; Orubu, E.S.F.; Wirtz, V.J.; Zaman, M.H. Bacterial antibiotic resistance development and mutagenesis following exposure to subminimal inhibitory concentrations of fluoroquinolones in vitro: A systematic literature review protocol. BMJ Open. 2019, 9, e030747. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin. Drug Discov. 2017, 12, 61–88. [Google Scholar] [CrossRef]
- Capasso, C.; Supuran, C.T. An overview of the selectivity and efficiency of the bacterial carbonic anhydrase inhibitors. Curr. Med. Chem. 2015, 22, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. Inhibition of Bacterial Carbonic Anhydrases as a Novel Approach to Escape Drug Resistance. Curr top Med Chem. 2017, 17, 1237–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supuran, C.T.; Capasso, C. An Overview of the Bacterial Carbonic Anhydrases. Metabolites 2017, 7, E56. [Google Scholar] [CrossRef] [Green Version]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem, J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef]
- Aspatwar, A.; Kairys, V.; Rala, S.; Parikka, M.; Bozdag, M.; Carta, F.; Supuran, C.T.; Parkkila, S. Mycobacterium tuberculosis β-Carbonic Anhydrases: Novel Targets for Developing Antituberculosis Drugs. Int. J. Mol. Sci. 2019, 20, E5153. [Google Scholar] [CrossRef] [Green Version]
- Angeli, A.; Pinteala, M.; Maier, S.S.; Del Prete, S.; Capasso, C.; Simionescu, B.C.; Supuran, C.T. Inhibition of bacterial α-, β- and γ-class carbonic anhydrases with selenazoles incorporating benzenesulfonamide moieties. J. Enzyme. Inhib. Med. Chem. 2019, 34, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Capasso, C.; Supuran, C.T. Anti-infective carbonic anhydrase inhibitors: A patent and literature review. Expert Opin. Ther. Pat. 2013, 23, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. An overview of the alpha-, beta- and gamma-carbonic anhydrases from Bacteria: Can bacterial carbonic anhydrases shed new light on evolution of bacteria? J. Enzyme. Inhib. Med. Chem. 2015, 30, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angeli, A.; Abbas, G.; Del Prete, S.; Carta, F.; Capasso, C.; Supuran, C.T. Acyl selenoureido benzensulfonamides show potent inhibitory activity against carbonic anhydrases from the pathogenic bacterium Vibrio cholerae. Bioorg. Chem. 2017, 75, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Angeli, A.; Abbas, G.; Del Prete, S.; Capasso, C.; Supuran, C.T. Selenides bearing benzenesulfonamide show potent inhibition activity against carbonic anhydrases from pathogenic bacteria Vibrio cholerae and Burkholderia pseudomallei. Bioorg. Chem. 2018, 79, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Alafeefy, A.M.; Ceruso, M.; Al-Tamimi, A.-M.S.; Del Prete, S.; Capasso, C.; Supuran, C.T. Quinazoline-sulfonamides with potent inhibitory activity against the α-carbonic anhydrase from Vibrio cholerae. Bioorg. Med. Chem. 2014, 22, 5133. [Google Scholar] [CrossRef] [PubMed]
- Tanini, D.; Capperucci, A.; Scopelliti, M.; Milaneschi, A.; Angeli, A.; Supuran, C.T. Syntesis of thio- and seleno-acetamides bearing benzenesulfonamide as potent inhibitors of human carbonic anhydrase II and XII. Bioorg. Chem. 2019, 89, 102984. [Google Scholar] [CrossRef]
- Tanini, D.; Scarpelli, S.; Ermini, E.; Capperucci, A. Seleno-Michael reaction of stable functionalised alkyl selenols: A versatile tool for the synthesis of acyclic and cyclic unsymmetrical alkyl and vinyl selenides. Adv. Synth. Catal. 2019, 361, 2337–2346. [Google Scholar] [CrossRef]
- Tanini, D.; Lupori, B.; Malevolti, G.; Ambrosi, M.; Lo Nostro, P.; Capperucci, A. Direct biocatalysed synthesis of first sulfur-, selenium- and tellurium containing L-ascorbyl hybrid derivatives with radical trapping and GPx-like properties. Chem. Commun. 2019, 55, 5705–5708. [Google Scholar] [CrossRef] [Green Version]
- Tanini, D.; Capperucci, A. Ring opening reactions of heterocycles with selenium and tellurium nucleophiles. New. J. Chem. 2019, 43, 11451–11468. [Google Scholar] [CrossRef] [Green Version]
- Mloston, G.; Capperucci, A.; Tanini, D.; Hamera-Faldyga, R.; Heimgartner, H. Dialkyl Dicyanofumarates as Oxidizing Reagents for the Conversion of Thiols into Disulfides and Selenols into Diselenides. Eur. J. Org. Chem. 2017, 46, 6831–6839. [Google Scholar] [CrossRef] [Green Version]
- Salvatore, R.N.; Smith, R.A.; Nischwitz, A.K.; Gavin, T. A mild and highly convenient chemoselective alkylation of thiols using Cs2CO3-TBAI. Tetrahedron Lett. 2005, 46, 8931–8935. [Google Scholar] [CrossRef]
- Tanini, D.; Tiberi, C.; Gellini, C.; Salvi, P.R.; Capperucci, A. A Straightforward access to stable β-functionalized alkyl selenols. Adv. Synth. Catal. 2018, 360, 3367–3375. [Google Scholar] [CrossRef]
- Khalifah, R.G. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971, 246, 2561. [Google Scholar]
- Obijalska, E.; Pawelec, M.; Mlostoń, G.; Capperucci, A.; Tanini, D.; Heimgartner, H. A Remarkable Influence of the Trifluoromethyl Group on the Reactions of β-Mercaptoalcohols with Fluorinated α-Bromoenones. Eur. J. Org. Chem. 2018, 3716–3723. [Google Scholar] [CrossRef]
- Angeli, A.; Tanini, D.; Nocentini, A.; Capperucci, A.; Ferraroni, M.; Gratteri, P.; Supuran, C.T. Selenols: A new class of carbonic anhydrase inhibitors. Chem. Commun. 2019, 55, 648–651. [Google Scholar] [CrossRef]
- Da’dara, A.A.; Angeli, A.; Ferraroni, M.; Supuran, C.T.; Skelly, P.J. Crystal structure and chemical inhibition of essential schistosome host-interactive virulence factor carbonic anhydrase SmCA. Commun. Biol. 2019, 2, 333. [Google Scholar] [CrossRef] [Green Version]
- Angeli, A.; Tanini, D.; Capperucci, A.; Supuran, C.T. First evaluation of organotellurium derivatives as carbonic anhydrase I, II, IV, VII and IX inhibitors. Bioorg. Chem. 2018, 76, 268–272. [Google Scholar] [CrossRef]
- Angeli, A.; Tanini, D.; Capperucci, A.; Supuran, C.T. Synthesis of Novel Selenides Bearing Benzenesulfonamide Moieties as Carbonic Anhydrase I, II, IV, VII, and IX inhibitors. ACS Med. Chem. Lett. 2017, 8, 1213–1217. [Google Scholar] [CrossRef]
- Angeli, A.; Di Cesare Mannelli, L.; Trallori, E.; Peat, T.S.; Ghelardini, C.; Carta, F.; Supuran, C.T. Design, Synthesis and X-ray of Selenides as New Class of Agents for Prevention of Diabetic Cerebrovascular Pathology. ACS Med. Chem. Lett. 2018, 9, 462–467. [Google Scholar]
- Angeli, A.; Carta, F.; Bartolucci, G.; Supuran, C.T. Synthesis of novel acyl selenoureido benzensulfonamides as carbonic anhydrase I, II, VII and IX inhibitors. Bioorg. Med. Chem. 2017, 25, 3567. [Google Scholar]
- Angeli, A.; Peat, T.S.; Bartolucci, G.; Nocentini, A.; Supuran, C.T.; Carta, F. Intramolecular oxidative deselenization of acylselenoureas: A facile synthesis of benzoxazole amides and carbonic anhydrase inhibitors. Org. Biomol. Chem. 2016, 14, 11353–11356. [Google Scholar] [CrossRef] [PubMed]



| KI (nM) * | ||||||
|---|---|---|---|---|---|---|
| Cmp | VchCA-α | VchCA-β | StCA2-β | BpsCA-β | BpsCA-γ | Rv3273-β |
| 3a | 49.9 | 4648 | 797.9 | >10,000 | 4168 | 2779 |
| 3b | 46.7 | 700.0 | 85.6 | >10,000 | 2750 | 1833 |
| 3c | 33.5 | 865.0 | 84.1 | >10,000 | 404.7 | 269.8 |
| 3d | >10,000 | 581.4 | 752.7 | >10,000 | 3819 | 2546 |
| 3e | 44.7 | 6651 | 77.7 | >10,000 | 321.5 | 214.3 |
| 3f | 84.9 | >10,000 | 482.8 | >10,000 | >10,000 | >10,000 |
| 4a | 51.1 | 700.0 | 86.7 | >10,000 | 3515 | 2343 |
| 4b | >10,000 | 7187 | 881.1 | >10,000 | >10,000 | >10,000 |
| 5a | 829.1 | 6225 | 865.0 | >10,000 | 1517 | 1011 |
| 5b | 45.8 | 580.7 | 8.2 | >10,000 | >10,000 | >10,000 |
| 7a | 2.2 | 96.3 | 814.7 | 4094 | 303.8 | 202.5 |
| 7b | 7.7 | 762.2 | >10,000 | >10,000 | 424.2 | 282.8 |
| 7c | 41.7 | 6210 | 451.3 | >10,000 | >10,000 | >10,000 |
| 8a | 44.4 | 6590 | 86.9 | >10,000 | 2250 | 1500 |
| 8b | 85.6 | 607.8 | 550.0 | >10,000 | 3634 | 2422 |
| 8c | 3.2 | 85.0 | 9.3 | >10,000 | >10,000 | >10,000 |
| 8d | 62.3 | 828.1 | 9.1 | >10,000 | >10,000 | >10,000 |
| 8e | 6.1 | 308.9 | 8.9 | >10,000 | >10,000 | >10,000 |
| 9a | 902.2 | 7468 | >10,000 | >10,000 | 449.7 | 299.8 |
| 9b | 88.6 | 729.5 | 27.3 | >10,000 | 4172 | 2781 |
| 9c | 730.3 | 7985 | 820.0 | >10,000 | 3656 | 2437 |
| 9d | 8.9 | 82.9 | 7.5 | >10,000 | >10,000 | >10,000 |
| AAZ | 6.8 | 451 | 89.0 | 745 | 149 | 104 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angeli, A.; Pinteala, M.; Maier, S.S.; Simionescu, B.C.; Milaneschi, A.; Abbas, G.; del Prete, S.; Capasso, C.; Capperucci, A.; Tanini, D.; et al. Evaluation of Thio- and Seleno-Acetamides Bearing Benzenesulfonamide as Inhibitor of Carbonic Anhydrases from Different Pathogenic Bacteria. Int. J. Mol. Sci. 2020, 21, 598. https://doi.org/10.3390/ijms21020598
Angeli A, Pinteala M, Maier SS, Simionescu BC, Milaneschi A, Abbas G, del Prete S, Capasso C, Capperucci A, Tanini D, et al. Evaluation of Thio- and Seleno-Acetamides Bearing Benzenesulfonamide as Inhibitor of Carbonic Anhydrases from Different Pathogenic Bacteria. International Journal of Molecular Sciences. 2020; 21(2):598. https://doi.org/10.3390/ijms21020598
Chicago/Turabian StyleAngeli, Andrea, Mariana Pinteala, Stelian S. Maier, Bogdan C. Simionescu, Andrea Milaneschi, Ghulam Abbas, Sonia del Prete, Clemente Capasso, Antonella Capperucci, Damiano Tanini, and et al. 2020. "Evaluation of Thio- and Seleno-Acetamides Bearing Benzenesulfonamide as Inhibitor of Carbonic Anhydrases from Different Pathogenic Bacteria" International Journal of Molecular Sciences 21, no. 2: 598. https://doi.org/10.3390/ijms21020598
APA StyleAngeli, A., Pinteala, M., Maier, S. S., Simionescu, B. C., Milaneschi, A., Abbas, G., del Prete, S., Capasso, C., Capperucci, A., Tanini, D., Carta, F., & Supuran, C. T. (2020). Evaluation of Thio- and Seleno-Acetamides Bearing Benzenesulfonamide as Inhibitor of Carbonic Anhydrases from Different Pathogenic Bacteria. International Journal of Molecular Sciences, 21(2), 598. https://doi.org/10.3390/ijms21020598