Ab Initio Study of the Interaction of a Graphene Surface Decorated with a Metal-Doped C30 with Carbon Monoxide, Carbon Dioxide, Methane, and Ozone
Abstract
:1. Introduction
2. Results
2.1. Optimization of the Semifullerene C30
2.2. Optimization of Graphene with a Six-Vacancy Cluster
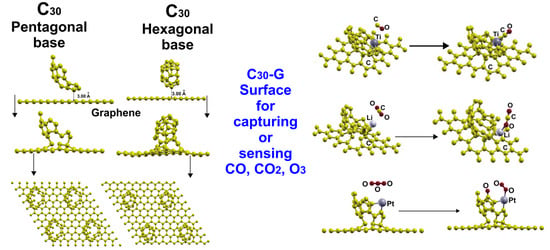
2.3. Adsorption of the C30 Molecule with a Pentagonal Base
2.4. Adsorption of the C30 Molecule with a Hexagonal Base
2.5. Adsorption of Metals on the Graphene-C30 (P) Surface
2.5.1. Doping with Li
2.5.2. Doping with Ti
2.5.3. Doping with Pt
2.6. Adsorption of Metals on the Graphene-C30 (H) Surface
2.6.1. Doping with Li
2.6.2. Doping with Ti
2.6.3. Doping with Pt
2.7. Adsorption of Pollutant Molecules on the Li-doped Graphene-C30 (P) Surface
2.7.1. Adsorption of CO
2.7.2. Adsorption of CO2
2.7.3. Adsorption of O3
2.8. Adsorption of Pollutant Molecules on the Ti-Doped Graphene-C30 (P) Surface
2.8.1. Adsorption of CO
2.8.2. Adsorption of CO2
2.8.3. Adsorption of CH4
2.8.4. Adsorption of O3
2.9. Adsorption of Pollutant Molecules on the Pt-Doped Graphene-C30 (P) Surface
2.9.1. Adsorption of CO
2.9.2. Adsorption of CO2
2.9.3. Adsorption of CH4
2.9.4. Adsorption of O3
2.10. Adsorption of Pollutant Molecules on the Li-Doped Graphene-C30 (H) Surface
2.10.1. Adsorption of CO
2.10.2. Adsorption of CO2
2.10.3. Adsorption of CH4
2.10.4. Adsorption of O3
2.11. Adsorption of Pollutant Molecules on the Ti-Doped Graphene-C30 (H) Surface
2.11.1. Adsorption of CO
2.11.2. Adsorption of CO2
2.11.3. Adsorption of CH4
2.11.4. Adsorption of O3
2.12. Adsorption of Pollutant Molecules on the Pt-Doped Graphene-C30 (H) Surface
2.12.1. Adsorption of CO
2.12.2. Adsorption of CO2
2.12.3. Adsorption of CH4
2.12.4. Adsorption of O3
3. Materials and Methods
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kjellstrom, T.; Lodh, M.; McMichael, T.; Ranmuthugala, G.; Shrestha, R.; Kingsland, S. Air and Water Pollution: Burden and Strategies for Control. In Disease Control Priorities in Developing Countries; Jamison, D.T., Breman, J.G., Measham, A.R., Alleyne, G., Claeson, M., Evans, D.B., Jha, P., Mills, A., Musgrove, P., Eds.; World Bank: Washington, DC, USA, 2006; ISBN 978-0-8213-6179-5. [Google Scholar]
- World Health Organization Don’t Pollute My Future! The Impact of the Environment on Children’s Health. Available online: https://www.who.int/publications-detail-redirect/WHO-FWC-IHE-17.01 (accessed on 12 April 2022).
- Hussain, A.; Rehman, F.; Rafeeq, H.; Waqas, M.; Asghar, A.; Afsheen, N.; Rahdar, A.; Bilal, M.; Iqbal, H.M.N. In-Situ, Ex-Situ, and Nano-Remediation Strategies to Treat Polluted Soil, Water, and Air–A Review. Chemosphere 2022, 289, 133252. [Google Scholar] [CrossRef]
- Osawa, E. Superaromaticity. Kagaku 1970, 25, 854–863. [Google Scholar]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Sygula, A. Chemistry on a Half-Shell: Synthesis and Derivatization of Buckybowls. Eur. J. Org. Chem. 2011, 2011, 1611–1625. [Google Scholar] [CrossRef]
- Astefanei, A.; Núñez, O.; Galceran, M.T. Characterisation and Determination of Fullerenes: A Critical Review. Anal. Chim. Acta 2015, 882, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Murayama, H.; Tomonoh, S.; Alford, J.M.; Karpuk, M.E. Fullerene Production in Tons and More: From Science to Industry. Fuller. Nanotub. Carbon Nanostruct. 2005, 12, 1–9. [Google Scholar] [CrossRef]
- Rabideau, P.W.; Abdourazak, A.H.; Folsom, H.E.; Marcinow, Z.; Sygula, A.; Sygula, R. Buckybowls: Synthesis and Ab Initio Calculated Structure of the First Semibuckminsterfullerene. J. Am. Chem. Soc. 1994, 116, 7891–7892. [Google Scholar] [CrossRef]
- Rabideau, P.W.; Sygula, A. Buckybowls: Polynuclear Aromatic Hydrocarbons Related to the Buckminsterfullerene Surface. Acc. Chem. Res. 1996, 29, 235–242. [Google Scholar] [CrossRef]
- Prinzbach, H.; Weiler, A.; Landenberger, P.; Wahl, F.; Wörth, J.; Scott, L.T.; Gelmont, M.; Olevano, D. Gas-Phase Production and Photoelectron Spectroscopy of the Smallest Fullerene, C20. Nature 2000, 407, 60–63. [Google Scholar] [CrossRef]
- Barth, W.E.; Lawton, R.G. Dibenzo[Ghi,Mno]Fluoranthene. J. Am. Chem. Soc. 1966, 88, 380–381. [Google Scholar] [CrossRef]
- Lawton, R.G.; Barth, W.E. Synthesis of Corannulene. J. Am. Chem. Soc. 1971, 93, 1730–1745. [Google Scholar] [CrossRef]
- Filatov, A.S.; Petrukhina, M.A. Probing the Binding Sites and Coordination Limits of Buckybowls in a Solvent-Free Environment: Experimental and Theoretical Assessment. Coord. Chem. Rev. 2010, 254, 2234–2246. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The Structure of Suspended Graphene Sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Boustani, I. New Quasi-Planar Surfaces of Bare Boron. Surf. Sci. 1997, 370, 355–363. [Google Scholar] [CrossRef]
- Švec, M.; Merino, P.; Dappe, Y.J.; González, C.; Abad, E.; Jelínek, P.; Martín-Gago, J.A. Van Der Waals Interactions Mediating the Cohesion of Fullerenes on Graphene. Phys. Rev. B 2012, 86, 121407. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Huang, Y.; Wang, Y.; Ma, Y.; Liu, Z.; Chen, Y. Synthesis and Characterization of a Graphene–C60 Hybrid Material. Carbon 2009, 47, 334–337. [Google Scholar] [CrossRef]
- Pu, J.; Mo, Y.; Wan, S.; Wang, L. Fabrication of Novel Graphene–Fullerene Hybrid Lubricating Films Based on Self-Assembly for MEMS Applications. Chem. Commun. 2013, 50, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, T.H.; Santos, E.J.G.; Jo, P.S.; Salleo, A.; Nishi, Y.; Bao, Z. Structural and Electrical Investigation of C 60 –Graphene Vertical Heterostructures. ACS Nano 2015, 9, 5922–5928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, H.; Wang, F.; Zhang, W.; Tang, S.; Ma, J.; Gong, H.; Zhang, J. Adsorption of Phosgene Molecule on the Transition Metal-Doped Graphene: First Principles Calculations. Appl. Surf. Sci. 2017, 425, 340–350. [Google Scholar] [CrossRef]
- Khodadadi, Z. Evaluation of H2S Sensing Characteristics of Metals–Doped Graphene and Metals-Decorated Graphene: Insights from DFT Study. Phys. E Low-Dimens. Syst. Nanostructures 2018, 99, 261–268. [Google Scholar] [CrossRef]
- Promthong, N.; Tabtimsai, C.; Rakrai, W.; Wanno, B. Transition Metal-Doped Graphene Nanoflakes for CO and CO2 Storage and Sensing Applications: A DFT Study. Struct. Chem. 2020, 31, 2237–2247. [Google Scholar] [CrossRef]
- Khan, A.A.; Ahmad, I.; Ahmad, R. Influence of Electric Field on CO2 Removal by P-Doped C60-Fullerene: A DFT Study. Chem. Phys. Lett. 2020, 742, 137155. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef] [Green Version]
- Kleinman, L.; Bylander, D.M. Efficacious Form for Model Pseudopotentials. Phys. Rev. Lett. 1982, 48, 1425–1428. [Google Scholar] [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Buongiorno Nardelli, M.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced Capabilities for Materials Modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, 81st ed.; CRC Press: Boca Raton, FL, USA, 2000; ISBN 978-0-8493-0481-1. [Google Scholar]
- Eyring, H. The Activated Complex in Chemical Reactions. J. Chem. Phys. 1935, 3, 107–115. [Google Scholar] [CrossRef]
- Popa, I.; Fernández, J.M.; Garcia-Manyes, S. Direct Quantification of the Attempt Frequency Determining the Mechanical Unfolding of Ubiquitin Protein. J. Biol. Chem. 2011, 286, 31072–31079. [Google Scholar] [CrossRef] [Green Version]






















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canales, M.; Ramírez-de-Arellano, J.M.; Arellano, J.S.; Magaña, L.F. Ab Initio Study of the Interaction of a Graphene Surface Decorated with a Metal-Doped C30 with Carbon Monoxide, Carbon Dioxide, Methane, and Ozone. Int. J. Mol. Sci. 2022, 23, 4933. https://doi.org/10.3390/ijms23094933
Canales M, Ramírez-de-Arellano JM, Arellano JS, Magaña LF. Ab Initio Study of the Interaction of a Graphene Surface Decorated with a Metal-Doped C30 with Carbon Monoxide, Carbon Dioxide, Methane, and Ozone. International Journal of Molecular Sciences. 2022; 23(9):4933. https://doi.org/10.3390/ijms23094933
Chicago/Turabian StyleCanales, Mónica, Juan Manuel Ramírez-de-Arellano, Juan Salvador Arellano, and Luis Fernando Magaña. 2022. "Ab Initio Study of the Interaction of a Graphene Surface Decorated with a Metal-Doped C30 with Carbon Monoxide, Carbon Dioxide, Methane, and Ozone" International Journal of Molecular Sciences 23, no. 9: 4933. https://doi.org/10.3390/ijms23094933
APA StyleCanales, M., Ramírez-de-Arellano, J. M., Arellano, J. S., & Magaña, L. F. (2022). Ab Initio Study of the Interaction of a Graphene Surface Decorated with a Metal-Doped C30 with Carbon Monoxide, Carbon Dioxide, Methane, and Ozone. International Journal of Molecular Sciences, 23(9), 4933. https://doi.org/10.3390/ijms23094933