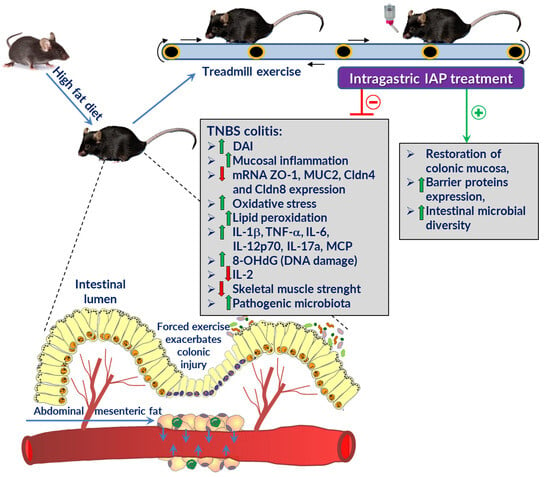
Alkaline Phosphatase Relieves Colitis in Obese Mice Subjected to Forced Exercise via Its Anti-Inflammatory and Intestinal Microbiota-Shaping Properties
Abstract
:1. Introduction
2. Results
2.1. Effect of IAP Treatment on Disease Activity Index (DAI), Body Weight and Relative Grip Strength in High-Fat Diet (HFD)- and Standard Diet (SD)-Fed Mice with Trinitrobenzene Sulfonic Acid (TNBS)-Induced Colitis Subjected to Forced Exercise on a Treadmill (T)
2.2. The Effect of Forced Treadmill Exercise Combined with IAP Administration on the Macroscopic and Microscopic Appearance of Colonic Mucosa in HFD- and SD-Fed Mice with Colitis
2.3. Effects of Forced Exercise Effort Combined with IAP Administration on the Mucosal Colonic Content of 8-Hydroxy-2′-Deoxyguanosine (8 OHdG), Malondialdehyde and 4-Hydroxynonenal (MDA+4-HNE), Total Glutathione (GSH+GSSG) Concentration and Superoxide Dismutase (SOD) Activity in HFD- or SD-Fed Mice with TNBS Colitis
2.4. Plasma Levels of Pro- and Anti-Inflammatory Cytokines in Exercising TNBS Colitis Mice Fed SD or HFD with or without IAP Treatment
2.5. The Alterations in mRNA Expression of Intestinal Barrier Proteins in Colonic Mucosa of Obese and Normal-Weight Exercising Mice with Experimental Colitis Administered with IAP
2.6. Changes in Gut Microbiome of Treadmill-Exercising Mice with TNBS Colitis Fed an SD or HFD with or without IAP Administration
3. Discussion
4. Materials and Methods
4.1. Animals and Diets
4.2. Experimental Design
- (1)
- Mice kept on a standard diet (SD), subjected to forced exercise on treadmill (T) and with colitis induced by 2,4,6-trinitrobenzenesulfonic acid (TNBS) (SD + T + TNBS);
- (2)
- Mice kept on a standard diet, subjected to forced exercise on treadmill, administered with intestinal alkaline phosphatase (IAP) and with induced colitis (SD + T + IAP + TNBS);
- (3)
- Mice fed a high-fat diet (HFD), subjected to forced exercise on treadmill and with induced colitis (HFD + T + TNBS).
- (4)
- Mice fed a high-fat diet, subjected to forced exercise on treadmill, administered with IAP followed by colitis induction (HFD + T + IAP + TNBS).
4.3. Grip Strength Test
4.4. Experimental Colitis Induction
4.5. Macroscopic and Microscopic Changes in the Colonic Mucosa Assessment
4.6. Next Generation Sequencing of Gut Microbiome
4.7. Determination of DNA Oxidation Levels by 8-Hydroxy-2′-Deoxyguanosine (8-OHdG) Concentration
4.8. Lipid Peroxidation Determination
4.9. Total Glutathione (GSH+GSSG) Concentration Measurement
4.10. Superoxide Dismutase (SOD) Activity Determination
4.11. Luminex Microbeads Fluorescent Assay and mRNA gene expression of intestinal barrier proteins ZO-1, MUC-2, Cldn-4 and Cldn-8
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaya, D.R.; Lyon, T.D.; Duncan, A.; Neilly, J.B.; Han, S.; Howell, J.; Liddell, C.; Stanley, A.J.; Morris, A.J.; Mackenzie, J.F. Faecal calprotectin in the assessment of Crohn’s disease activity. QJM 2005, 98, 435–441. [Google Scholar] [CrossRef]
- Hanauer, S.B. Inflammatory bowel disease: Epidemiology, pathogenesis, and therapeutic opportunities. Inflamm. Bowel Dis. 2006, 12 (Suppl. S1), S3–S9. [Google Scholar] [CrossRef]
- Shanahan, F. Crohn’s disease. Lancet 2002, 359, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Limdi, J.K.; Picco, M.; Farraye, F.A. A review of endoscopic scoring systems and their importance in a treat-to-target approach in inflammatory bowel disease (with videos). Gastrointest. Endosc. 2020, 91, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, C.F.; Yann, L.H.; Lal, S. Nutritional management of Crohn’s disease. Ther. Adv. Gastroenterol. 2013, 6, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Seminerio, J.L.; Koutroubakis, I.E.; Ramos-Rivers, C.; Hashash, J.G.; Dudekula, A.; Regueiro, M.; Baidoo, L.; Barrie, A.; Swoger, J.; Schwartz, M.; et al. Impact of Obesity on the Management and Clinical Course of Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 2857–2863. [Google Scholar] [CrossRef]
- Fink, C.; Karagiannides, I.; Bakirtzi, K.; Pothoulakis, C. Adipose tissue and inflammatory bowel disease pathogenesis. Inflamm. Bowel Dis. 2012, 18, 1550–1557. [Google Scholar] [CrossRef]
- Bilski, J.; Brzozowski, B.; Mazur-Bialy, A.; Sliwowski, Z.; Brzozowski, T. The role of physical exercise in inflammatory bowel disease. Biomed. Res. Int. 2014, 2014, 429031. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Bilski, J.; Wojcik, D.; Brzozowski, B.; Surmiak, M.; Hubalewska-Mazgaj, M.; Chmura, A.; Magierowski, M.; Magierowska, K.; Mach, T.; et al. Beneficial Effect of Voluntary Exercise on Experimental Colitis in Mice Fed a High-Fat Diet: The Role of Irisin, Adiponectin and Proinflammatory Biomarkers. Nutrients 2017, 9, 410. [Google Scholar] [CrossRef]
- Wojcik-Grzybek, D.; Hubalewska-Mazgaj, M.; Surmiak, M.; Sliwowski, Z.; Dobrut, A.; Mlodzinska, A.; Wojcik, A.; Kwiecien, S.; Magierowski, M.; Mazur-Bialy, A.; et al. The Combination of Intestinal Alkaline Phosphatase Treatment with Moderate Physical Activity Alleviates the Severity of Experimental Colitis in Obese Mice via Modulation of Gut Microbiota, Attenuation of Proinflammatory Cytokines, Oxidative Stress Biomarkers and DNA Oxidative Damage in Colonic Mucosa. Int. J. Mol. Sci. 2022, 23, 2964. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Snipe, R.M.J.; Kitic, C.M.; Gibson, P.R. Systematic review: Exercise-induced gastrointestinal syndrome-implications for health and intestinal disease. Aliment. Pharmacol. Ther. 2017, 46, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Mavrelis, P.G.; Georgis, P.; Todd, M.; Gabrys, S. Exercise-Induced Ischemic Colitis from Competitive Cycling. Curr. Sports Med. Rep. 2021, 20, 575–576. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.C.; Winstanley, A.; Engledow, A.; Windsor, A.C.; Skipworth, J.R. Marathon-induced ischemic colitis: Why running is not always good for you. Am. J. Emerg. Med. 2009, 27, e255–e257. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Mazur-Bialy, A.; Wojcik, D.; Magierowski, M.; Surmiak, M.; Kwiecien, S.; Magierowska, K.; Hubalewska-Mazgaj, M.; Sliwowski, Z.; Brzozowski, T. Effect of Forced Physical Activity on the Severity of Experimental Colitis in Normal Weight and Obese Mice. Involvement of Oxidative Stress and Proinflammatory Biomarkers. Nutrients 2019, 11, 1127. [Google Scholar] [CrossRef]
- Gao, X.; Cao, Q.; Cheng, Y.; Zhao, D.; Wang, Z.; Yang, H.; Wu, Q.; You, L.; Wang, Y.; Lin, Y.; et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc. Natl. Acad. Sci. USA 2018, 115, E2960–E2969. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Mazur-Bialy, A.; Wojcik, D.; Surmiak, M.; Magierowski, M.; Sliwowski, Z.; Pajdo, R.; Kwiecien, S.; Danielak, A.; Ptak-Belowska, A.; et al. Role of Obesity, Mesenteric Adipose Tissue, and Adipokines in Inflammatory Bowel Diseases. Biomolecules 2019, 9, 780. [Google Scholar] [CrossRef]
- Konturek, P.C.; Konturek, K.; Brzozowski, T.; Wojcik, D.; Magierowski, M.; Targosz, A.; Krzysiek-Maczka, G.; Sliwowski, Z.; Strzalka, M.; Magierowska, K.; et al. Participation of the intestinal microbiota in the mechanism of beneficial effect of treatment with synbiotic Syngut on experimental colitis under stress conditions. J. Physiol. Pharmacol. 2020, 71, 329–342. [Google Scholar] [CrossRef]
- Danielak, A.; Wojcik, D.; Mazur-Bialy, A.; Surmiak, M.; Bilski, J.; Targosz, A.; Magierowski, M.; Chmura, A.; Strzalka, M.; Krzysiek-Maczka, G.; et al. Intestinal Alkaline Phosphatase Combined with Voluntary Physical Activity Alleviates Experimental Colitis in Obese Mice. Involvement of Oxidative Stress, Myokines, Adipokines and Proinflammatory Biomarkers. Antioxidant 2021, 10, 240. [Google Scholar] [CrossRef]
- Moss, A.K.; Hamarneh, S.R.; Mohamed, M.M.; Ramasamy, S.; Yammine, H.; Patel, P.; Kaliannan, K.; Alam, S.N.; Muhammad, N.; Moaven, O.; et al. Intestinal alkaline phosphatase inhibits the proinflammatory nucleotide uridine diphosphate. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G597–G604. [Google Scholar] [CrossRef]
- Shifrin, D.A., Jr.; McConnell, R.E.; Nambiar, R.; Higginbotham, J.N.; Coffey, R.J.; Tyska, M.J. Enterocyte microvillus-derived vesicles detoxify bacterial products and regulate epithelial-microbial interactions. Curr. Biol. 2012, 22, 627–631. [Google Scholar] [CrossRef]
- Liu, W.; Hu, D.; Huo, H.; Zhang, W.; Adiliaghdam, F.; Morrison, S.; Ramirez, J.M.; Gul, S.S.; Hamarneh, S.R.; Hodin, R.A. Intestinal Alkaline Phosphatase Regulates Tight Junction Protein Levels. J. Am. Coll. Surg. 2016, 222, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, M.B.; Dhawan, P.; Baumert, T.F. Tight junction proteins in gastrointestinal and liver disease. Gut 2019, 68, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Takechi, M.; Kiyonari, H.; Shioi, G.; Tamura, A.; Tsukita, S. Intestinal deletion of Claudin-7 enhances paracellular organic solute flux and initiates colonic inflammation in mice. Gut 2015, 64, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhou, M.F.; Wang, Y.D.; Chen, L.P.; Xu, W.F.; Wang, Y.D.; Deng, F.; Liu, S.D. Protein Kinase D2 Protects against Acute Colitis Induced by Dextran Sulfate Sodium in Mice. Sci. Rep. 2016, 6, 34079. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Zhu, X.; Luo, X.; Feng, Y.; Wang, J. PIK3R3 regulates ZO-1 expression through the NF-kB pathway in inflammatory bowel disease. Int. Immunopharmacol. 2020, 85, 106610. [Google Scholar] [CrossRef]
- Lukas, M.; Drastich, P.; Konecny, M.; Gionchetti, P.; Urban, O.; Cantoni, F.; Bortlik, M.; Duricova, D.; Bulitta, M. Exogenous alkaline phosphatase for the treatment of patients with moderate to severe ulcerative colitis. Inflamm. Bowel Dis. 2010, 16, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Ateş, B.B.; Talim, B.; Gülşen, H.H.; Demir, H.; Karaismailoğlu, E.; Özen, H.; Saltık Temizel, İ.N. Significance of intestinal alkaline phosphatase in predicting histological activity of pediatric inflammatory bowel disease. Turk. J. Pediatr. 2022, 64, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Parlato, M.; Charbit-Henrion, F.; Pan, J.; Romano, C.; Duclaux-Loras, R.; Le Du, M.H.; Warner, N.; Francalanci, P.; Bruneau, J.; Bras, M.; et al. Human ALPI deficiency causes inflammatory bowel disease and highlights a key mechanism of gut homeostasis. EMBO Mol. Med. 2018, 10, e8483. [Google Scholar] [CrossRef]
- Tuin, A.; Poelstra, K.; de Jager-Krikken, A.; Bok, L.; Raaben, W.; Velders, M.P.; Dijkstra, G. Role of alkaline phosphatase in colitis in man and rats. Gut 2009, 58, 379–387. [Google Scholar] [CrossRef]
- Hwang, S.W.; Kim, J.H.; Lee, C.; Im, J.P.; Kim, J.S. Intestinal alkaline phosphatase ameliorates experimental colitis via toll-like receptor 4-dependent pathway. Eur. J. Pharmacol. 2018, 820, 156–166. [Google Scholar] [CrossRef]
- Molnar, K.; Vannay, A.; Sziksz, E.; Banki, N.F.; Gyorffy, H.; Arato, A.; Dezsofi, A.; Veres, G. Decreased mucosal expression of intestinal alkaline phosphatase in children with coeliac disease. Virchows Arch. 2012, 460, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Malo, M.S. A High Level of Intestinal Alkaline Phosphatase Is Protective Against Type 2 Diabetes Mellitus Irrespective of Obesity. EBioMedicine 2015, 2, 2016–2023. [Google Scholar] [CrossRef] [PubMed]
- Leung, G.; Muise, A.M. Monogenic Intestinal Epithelium Defects and the Development of Inflammatory Bowel Disease. Physiology 2018, 33, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Vercalsteren, E.; Vranckx, C.; Lijnen, H.R.; Hemmeryckx, B.; Scroyen, I. Adiposity and metabolic health in mice deficient in intestinal alkaline phosphatase. Adipocyte 2018, 7, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Backhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Laukens, D.; Brinkman, B.M.; Raes, J.; De Vos, M.; Vandenabeele, P. Heterogeneity of the gut microbiome in mice: Guidelines for optimizing experimental design. FEMS Microbiol. Rev. 2016, 40, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Armougom, F.; Million, M.; Raoult, D. The relationship between gut microbiota and weight gain in humans. Future Microbiol. 2012, 7, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS ONE 2014, 9, e92193. [Google Scholar] [CrossRef]
- Reunanen, J.; Kainulainen, V.; Huuskonen, L.; Ottman, N.; Belzer, C.; Huhtinen, H.; de Vos, W.M.; Satokari, R. Akkermansia muciniphila Adheres to Enterocytes and Strengthens the Integrity of the Epithelial Cell Layer. Appl. Environ. Microbiol. 2015, 81, 3655–3662. [Google Scholar] [CrossRef]
- Bamola, V.D.; Ghosh, A.; Kapardar, R.K.; Lal, B.; Cheema, S.; Sarma, P.; Chaudhry, R. Gut microbial diversity in health and disease: Experience of healthy Indian subjects, and colon carcinoma and inflammatory bowel disease patients. Microb. Ecol. Health Dis. 2017, 28, 1322447. [Google Scholar] [CrossRef]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Liu, H.; Yu, H.; Chen, M.; Yang, T.; Zeng, X.; Qiao, S. Core Altered Microorganisms in Colitis Mouse Model: A Comprehensive Time-Point and Fecal Microbiota Transplantation Analysis. Antibiotics 2021, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.F.; Souza, N.C.; Chiarello, P.G.; Franceschini, S.C.; Bressan, J.; Ferreira, C.L.; Peluzio Mdo, C. Intestinal permeability parameters in obese patients are correlated with metabolic syndrome risk factors. Clin. Nutr. 2012, 31, 735–740. [Google Scholar] [CrossRef]
- Teshima, C.W.; Dieleman, L.A.; Meddings, J.B. Abnormal intestinal permeability in Crohn’s disease pathogenesis. Ann. N. Y. Acad. Sci. 2012, 1258, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Borisova, M.A.; Achasova, K.M.; Morozova, K.N.; Andreyeva, E.N.; Litvinova, E.A.; Ogienko, A.A.; Morozova, M.V.; Berkaeva, M.B.; Kiseleva, E.; Kozhevnikova, E.N. Mucin-2 knockout is a model of intercellular junction defects, mitochondrial damage and ATP depletion in the intestinal epithelium. Sci. Rep. 2020, 10, 21135. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.M.; Ismael, S.; Morais, J.; Araujo, J.R.; Faria, A.; Calhau, C.; Marques, C. Intestinal Alkaline Phosphatase: A Review of This Enzyme Role in the Intestinal Barrier Function. Microorganisms 2022, 10, 746. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Hansson, G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef]
- Formiga, R.O.; Alves Junior, E.B.; Vasconcelos, R.C.; Guerra, G.C.B.; Antunes de Araujo, A.; Carvalho, T.G.; Garcia, V.B.; de Araujo Junior, R.F.; Gadelha, F.; Vieira, G.C.; et al. p-Cymene and Rosmarinic Acid Ameliorate TNBS-Induced Intestinal Inflammation Upkeeping ZO-1 and MUC-2: Role of Antioxidant System and Immunomodulation. Int. J. Mol. Sci. 2020, 21, 5870. [Google Scholar] [CrossRef]
- Wang, G.; Xu, Q.; Jin, X.; Hang, F.; Liu, Z.; Zhao, J.; Zhang, H.; Chen, W. Effects of lactobacilli with different regulatory behaviours on tight junctions in mice with dextran sodium sulphate-induced colitis. J. Funct. Foods 2018, 47, 107–115. [Google Scholar] [CrossRef]
- Kuo, W.T.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, W.; Wang, X.; Guo, F.; Cheng, Y.; Cao, L.; Zhu, W.; Sun, Y.; Xiong, H. Industrially Produced Rice Protein Ameliorates Dextran Sulfate Sodium-Induced Colitis via Protecting the Intestinal Barrier, Mitigating Oxidative Stress, and Regulating Gut Microbiota. J. Agric. Food Chem. 2022, 70, 4952–4965. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Iantomasi, T.; Bonanomi, A.G.; Stio, M. Vitamin D regulates claudin-2 and claudin-4 expression in active ulcerative colitis by p-Stat-6 and Smad-7 signaling. Int. J. Color. Dis. 2020, 35, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Mees, S.T.; Mennigen, R.; Spieker, T.; Rijcken, E.; Senninger, N.; Haier, J.; Bruewer, M. Expression of tight and adherens junction proteins in ulcerative colitis associated colorectal carcinoma: Upregulation of claudin-1, claudin-3, claudin-4, and beta-catenin. Int. J. Color. Dis. 2009, 24, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Barmeyer, C.; Erko, I.; Awad, K.; Fromm, A.; Bojarski, C.; Meissner, S.; Loddenkemper, C.; Kerick, M.; Siegmund, B.; Fromm, M. Epithelial barrier dysfunction in lymphocytic colitis through cytokine-dependent internalization of claudin-5 and-8. J. Gastroenterol. 2017, 52, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Manzo, L.P.; de-Faria, F.M.; Dunder, R.J.; Rabelo-Socca, E.A.; Consonni, S.R.; de Almeida, A.C.; Souza-Brito, A.R.; Luiz-Ferreira, A. Royal Jelly and its dual role in TNBS colitis in mice. Sci. World J. 2015, 2015, 956235. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Zhao, W.; Dou, Y.; An, H.; Tao, H.; Xu, X.; Jia, Y.; Lu, S.; Zhang, J.; et al. Targeted Therapy of Atherosclerosis by a Broad-Spectrum Reactive Oxygen Species Scavenging Nanoparticle with Intrinsic Anti-inflammatory Activity. ACS Nano 2018, 12, 8943–8960. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gao, W.; Cai, X.; Xu, J.; Zou, D.; Li, Z.; Hu, B.; Zheng, Y. Nanozyme-mediated catalytic nanotherapy for inflammatory bowel disease. Theranostics 2019, 9, 2843–2855. [Google Scholar] [CrossRef]
- Zhou, G.; Yu, L.; Fang, L.; Yang, W.; Yu, T.; Miao, Y.; Chen, M.; Wu, K.; Chen, F.; Cong, Y.; et al. CD177(+) neutrophils as functionally activated neutrophils negatively regulate IBD. Gut 2018, 67, 1052–1063. [Google Scholar] [CrossRef]
- Ock, C.Y.; Kim, E.H.; Hong, H.; Hong, K.S.; Han, Y.M.; Choi, K.S.; Hahm, K.B.; Chung, M.H. Prevention of colitis-associated colorectal cancer with 8-hydroxydeoxyguanosine. Cancer Prev. Res. 2011, 4, 1507–1521. [Google Scholar] [CrossRef]
- Ohira, H.; Tsuruya, A.; Oikawa, D.; Nakagawa, W.; Mamoto, R.; Hattori, M.; Waki, T.; Takahashi, S.; Fujioka, Y.; Nakayama, T. Alteration of oxidative-stress and related marker levels in mouse colonic tissues and fecal microbiota structures with chronic ethanol administration: Implications for the pathogenesis of ethanol-related colorectal cancer. PLoS ONE 2021, 16, e0246580. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, Y.; Zhang, H.; Zhou, M.; Yu, Y.; Lin, S.; Jiang, B.; Zhao, X.; Miao, L.; Wei, C.W.; et al. Integrated cascade nanozyme catalyzes in vivo ROS scavenging for anti-inflammatory therapy. Sci. Adv. 2020, 6, eabb2695. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Shao, X.; Tao, Y.; Wang, S.; Han, H.; Li, Q. Superoxide dismutase-embedded metal-organic frameworks via biomimetic mineralization for the treatment of inflammatory bowel disease. J. Mater. Chem. B 2022, 10, 5174–5181. [Google Scholar] [CrossRef] [PubMed]
- Kochumon, S.; Al Madhoun, A.; Al-Rashed, F.; Thomas, R.; Sindhu, S.; Al-Ozairi, E.; Al-Mulla, F.; Ahmad, R. Elevated adipose tissue associated IL-2 expression in obesity correlates with metabolic inflammation and insulin resistance. Sci. Rep. 2020, 10, 16364. [Google Scholar] [CrossRef] [PubMed]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kuhl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar] [PubMed]
- Glowacka, U.; Magierowska, K.; Wojcik, D.; Hankus, J.; Szetela, M.; Cieszkowski, J.; Korbut, E.; Danielak, A.; Surmiak, M.; Chmura, A.; et al. Microbiome Profile and Molecular Pathways Alterations in Gastrointestinal Tract by Hydrogen Sulfide-Releasing Nonsteroidal Anti-Inflammatory Drug (ATB-352): Insight into Possible Safer Polypharmacy. Antioxid. Redox Signal. 2022, 36, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Fernandes, A.D.; Reid, J.N.; Macklaim, J.M.; McMurrough, T.A.; Edgell, D.R.; Gloor, G.B. Unifying the analysis of high-throughput sequencing datasets: Characterizing RNA-seq, 16S rRNA gene sequencing and selective growth experiments by compositional data analysis. Microbiome 2014, 2, 15. [Google Scholar] [CrossRef]
- Magierowska, K.; Korbut, E.; Hubalewska-Mazgaj, M.; Surmiak, M.; Chmura, A.; Bakalarz, D.; Buszewicz, G.; Wojcik, D.; Sliwowski, Z.; Ginter, G.; et al. Oxidative gastric mucosal damage induced by ischemia/reperfusion and the mechanisms of its prevention by carbon monoxide-releasing tricarbonyldichlororuthenium (II) dimer. Free Radic. Biol. Med. 2019, 145, 198–208. [Google Scholar] [CrossRef]
- Magierowska, K.; Bakalarz, D.; Wojcik, D.; Korbut, E.; Danielak, A.; Glowacka, U.; Pajdo, R.; Buszewicz, G.; Ginter, G.; Surmiak, M.; et al. Evidence for Cytoprotective Effect of Carbon Monoxide Donor in the Development of Acute Esophagitis Leading to Acute Esophageal Epithelium Lesions. Cells 2020, 9, 1203. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: Twenty-something years on. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Marincola Smith, P.; Choksi, Y.A.; Markham, N.O.; Hanna, D.N.; Zi, J.; Weaver, C.J.; Hamaamen, J.A.; Lewis, K.B.; Yang, J.; Liu, Q.; et al. Colon epithelial cell TGFbeta signaling modulates the expression of tight junction proteins and barrier function in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G936–G957. [Google Scholar] [CrossRef] [PubMed]









| PCR Product | Primer Sequence | Product Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| b-actin | Forward: 5′-CCCATCTATGAGGGTTACGC-3′ Reverse: 5′-TTTAATGTCACGCACGATTTC-3′ | 150 | 60 |
| ZO-1 | Forward: 5′-GTTGGTACGGTGCCCTGAAAGA-3′ Reverse: 5′-GCTGACAGGTAGGACAGACGAT-3′ | 133 | 60 |
| Muc2 | Forward: 5′-TCCTGACCAAGAGCGAACAC-3′ Reverse: 5′-GGGTAGGGTCACCTCCATCT-3′ | 182 | 60 |
| Cldn4 | Forward: 5′-TTTTGTGGTCACCGACTTTG-3′ Reverse: 5′-TGTAGTCCCATAGACGCCATC-3′ | 60 | 60 |
| Cldn8 | Forward: 5′-GGGCCTGGGGATAAAAGAG-3′ Reverse: 5′-AATCCTTAAGCTGTTTTTAGGCAAT-3′ | 60 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojcik-Grzybek, D.; Sliwowski, Z.; Kwiecien, S.; Ginter, G.; Surmiak, M.; Hubalewska-Mazgaj, M.; Chmura, A.; Wojcik, A.; Kosciolek, T.; Danielak, A.; et al. Alkaline Phosphatase Relieves Colitis in Obese Mice Subjected to Forced Exercise via Its Anti-Inflammatory and Intestinal Microbiota-Shaping Properties. Int. J. Mol. Sci. 2024, 25, 703. https://doi.org/10.3390/ijms25020703
Wojcik-Grzybek D, Sliwowski Z, Kwiecien S, Ginter G, Surmiak M, Hubalewska-Mazgaj M, Chmura A, Wojcik A, Kosciolek T, Danielak A, et al. Alkaline Phosphatase Relieves Colitis in Obese Mice Subjected to Forced Exercise via Its Anti-Inflammatory and Intestinal Microbiota-Shaping Properties. International Journal of Molecular Sciences. 2024; 25(2):703. https://doi.org/10.3390/ijms25020703
Chicago/Turabian StyleWojcik-Grzybek, Dagmara, Zbigniew Sliwowski, Slawomir Kwiecien, Grzegorz Ginter, Marcin Surmiak, Magdalena Hubalewska-Mazgaj, Anna Chmura, Adrianna Wojcik, Tomasz Kosciolek, Aleksandra Danielak, and et al. 2024. "Alkaline Phosphatase Relieves Colitis in Obese Mice Subjected to Forced Exercise via Its Anti-Inflammatory and Intestinal Microbiota-Shaping Properties" International Journal of Molecular Sciences 25, no. 2: 703. https://doi.org/10.3390/ijms25020703
APA StyleWojcik-Grzybek, D., Sliwowski, Z., Kwiecien, S., Ginter, G., Surmiak, M., Hubalewska-Mazgaj, M., Chmura, A., Wojcik, A., Kosciolek, T., Danielak, A., Targosz, A., Strzalka, M., Szczyrk, U., Ptak-Belowska, A., Magierowski, M., Bilski, J., & Brzozowski, T. (2024). Alkaline Phosphatase Relieves Colitis in Obese Mice Subjected to Forced Exercise via Its Anti-Inflammatory and Intestinal Microbiota-Shaping Properties. International Journal of Molecular Sciences, 25(2), 703. https://doi.org/10.3390/ijms25020703