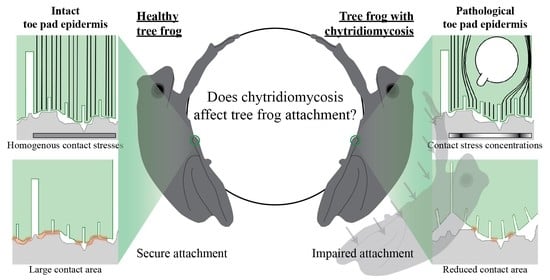
Does Chytridiomycosis Affect Tree Frog Attachment?
Abstract
:1. Introduction
2. Comparing the Morphology of Healthy and Bd-Infected Anuran Epidermis
2.1. Functional Morphology of Tree Frog Toe Pads
2.2. Epidermal Morphology of Bd-Infected Frogs
3. Potential Sublethal Effects of Chytridiomycosis on Tree Frog Attachment
3.1. Mechanisms of Tree Frog Attachment
3.2. Bd-Induced Disruptions of Tree Frog Attachment
4. How to Test If Bd Affects Tree Frog Attachment?
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.L.; Fischman, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Scheele, B.C.; Pasmans, F.; Skerratt, L.F.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A.; et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef]
- Lambert, M.R.; Womack, M.C.; Byrne, A.Q.; Hernández-Gómez, O.; Noss, C.F.; Rothstein, A.P.; Blackburn, D.C.; Collins, J.P.; Crump, M.L.; Koo, M.S.; et al. Comment on “Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity”. Science 2020, 367, eaay1838. [Google Scholar] [CrossRef] [Green Version]
- Brito, D. Amphibian conservation: Are we on the right track? Biol. Conserv. 2008, 141, 2912–2917. [Google Scholar] [CrossRef]
- Ford, J.; Hunt, D.A.G.A.; Haines, G.E.; Lewis, M.; Lewis, Y.; Green, D.M. Adrift on a sea of troubles: Can amphibians survive in a human-dominated world? Herpetologica 2020, 76, 251. [Google Scholar] [CrossRef]
- Van Rooij, P.; Martel, A.; Haesebrouck, F.; Pasmans, F. Amphibian chytridiomycosis: A review with focus on fungus-host interactions. Vet. Res. 2015, 46, 137. [Google Scholar] [CrossRef] [Green Version]
- Berger, L.; Speare, R.; Daszak, P.; Green, D.E.; Cunningham, A.A.; Goggin, C.L.; Slocombe, R.; Ragan, M.A.; Hyatt, A.D.; McDonald, K.R.; et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl. Acad. Sci. USA 1998, 95, 9031–9036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, L.; Speare, R.; Skerratt, L. Distribution of Batrachochytrium dendrobatidis and pathology in the skin of green tree frogs Litoria caerulea with severe chytridiomycosis. Dis. Aquat. Organ. 2005, 68, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, J.F.A.; Bui-Marinos, M.P.; Katzenback, B.A. Frog skin innate immune defences: Sensing and surviving pathogens. Front. Immunol. 2019, 9, 3128. [Google Scholar] [CrossRef] [PubMed]
- Voyles, J.; Berger, L.; Young, S.; Speare, R.; Webb, R.; Warner, J.; Rudd, D.; Campbell, R.; Skerratt, L. Electrolyte depletion and osmotic imbalance in amphibians with chytridiomycosis. Dis. Aquat. Organ. 2007, 77, 113–118. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Briggs, C.J.; Daszak, P. The ecology and impact of chytridiomycosis: An emerging disease of amphibians. Trends Ecol. Evol. 2010, 25, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.E.; Halstead, B.J.; Mosher, B.A.; Muths, E.; Adams, M.J.; Grant, E.H.C.; Fisher, R.N.; Kleeman, P.M.; Backlin, A.R.; Pearl, C.A.; et al. Effect of amphibian chytrid fungus (Batrachochytrium dendrobatidis) on apparent survival of frogs and toads in the western USA. Biol. Conserv. 2019, 236, 296–304. [Google Scholar] [CrossRef]
- Bielby, J.; Fisher, M.C.; Clare, F.C.; Rosa, G.M.; Garner, T.W.J. Host species vary in infection probability, sub-lethal effects and costs of immune response when exposed to an amphibian parasite. Sci. Rep. 2015, 5, 10828. [Google Scholar] [CrossRef] [Green Version]
- Brannelly, L.A.; Chatfield, M.W.H.; Sonn, J.; Robak, M.; Richards-Zawacki, C.L. Fungal infection has sublethal effects in a lowland subtropical amphibian population. BMC Ecol. 2018, 18, 34. [Google Scholar] [CrossRef] [Green Version]
- Ohmer, M.E.B.; Cramp, R.L.; White, C.R.; Franklin, C.E. Skin sloughing rate increases with chytrid fungus infection load in a susceptible amphibian. Funct. Ecol. 2015, 29, 674–682. [Google Scholar] [CrossRef] [Green Version]
- Ohmer, M.E.B.; Cramp, R.L.; Russo, C.J.M.; White, C.R.; Franklin, C.E. Skin sloughing in susceptible and resistant amphibians regulates infection with a fungal pathogen. Sci. Rep. 2017, 7, 3529. [Google Scholar] [CrossRef] [Green Version]
- Russo, C.J.M.; Ohmer, M.E.B.; Cramp, R.L.; Franklin, C.E. A pathogenic skin fungus and sloughing exacerbate cutaneous water loss in amphibians. J. Exp. Biol. 2018, 221, jeb167445. [Google Scholar] [CrossRef] [Green Version]
- Joseph, M.B.; Knapp, R.A. Disease and climate effects on individuals drive post-reintroduction population dynamics of an endangered amphibian. Ecosphere 2018, 9, e02499. [Google Scholar] [CrossRef] [Green Version]
- Spitzen-van der Sluijs, A.; Canessa, S.; Martel, A.; Pasmans, F. Fragile coexistence of a global chytrid pathogen with amphibian populations is mediated by environment and demography. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171444. [Google Scholar] [CrossRef] [PubMed]
- Bienentreu, J.-F.; Lesbarrères, D. Amphibian disease ecology: Are we just scratching the surface? Herpetologica 2020, 76, 153. [Google Scholar] [CrossRef]
- Cartmill, M. Climbing. In Functional Vertebrate Morphology; Hildebrand, M., Bramble, D.M., Liem, K.F., Wake, D.B., Eds.; Harvard University Press: Cambridge, MA, USA, 1985; pp. 73–88. [Google Scholar]
- Puschendorf, R.; Bolaños, F. Detection of Batrachochytrium dendrobatides in Eleutherodactylus fitzingeri: Effects of skin sample location and histological stain. J. Wildl. Dis. 2006, 42, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Manzano, A.S.; Fabrezi, M.; Vences, M. Intercalary elements, treefrogs, and the early differentiation of a complex system in the Neobatrachia. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2007, 290, 1551–1567. [Google Scholar] [CrossRef] [PubMed]
- Endlein, T.; Ji, A.; Samuel, D.; Yao, N.; Wang, Z.; Barnes, W.J.P.; Federle, W.; Kappl, M.; Dai, Z. Sticking like sticky tape: Tree frogs use friction forces to enhance attachment on overhanging surfaces. J. R. Soc. Interface 2013, 10, 20120838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzano, A.S.; Fontanarrosa, G.; Abdala, V. Manual and pedal grasping among anurans: A review of relevant concepts with empirical approaches. Biol. J. Linn. Soc. 2019, 127, 598–610. [Google Scholar] [CrossRef]
- Manzano, A.S.; Abdala, V.; Herrel, A. Morphology and function of the forelimb in arboreal frogs: Specializations for grasping ability? J. Anat. 2008, 213, 296–307. [Google Scholar] [CrossRef] [Green Version]
- Sustaita, D.; Pouydebat, E.; Manzano, A.; Abdala, V.; Hertel, F.; Herrel, A. Getting a grip on tetrapod grasping: Form, function, and evolution. Biol. Rev. 2013, 88, 380–405. [Google Scholar] [CrossRef]
- Langowski, J.K.A.; Dodou, D.; Kamperman, M.; van Leeuwen, J.L. Tree frog attachment: Mechanisms, challenges, and perspectives. Front. Zool. 2018, 15, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langowski, J.K.A.; Dodou, D.; van Assenbergh, P.; van Leeuwen, J.L. Design of tree-frog-inspired adhesives. Integr. Comp. Biol. 2020, 60, 906–918. [Google Scholar] [CrossRef]
- Ernst, V.V. The digital pads of the tree frog, Hyla cinerea. I. The epidermis. Tissue Cell 1973, 5, 83–96. [Google Scholar] [CrossRef]
- Ernst, V.V. The digital pads of the tree frog, Hyla cinerea. II. The mucous glands. Tissue Cell 1973, 5, 97–104. [Google Scholar] [CrossRef]
- Langowski, J.K.A.; Schipper, H.; Blij, A.; van den Berg, F.T.; Gussekloo, S.W.S.; van Leeuwen, J.L. Force-transmitting structures in the digital pads of the tree frog Hyla cinerea: A functional interpretation. J. Anat. 2018, 233, 478–495. [Google Scholar] [CrossRef] [PubMed]
- Federle, W.; Barnes, W.J.; Baumgartner, W.; Drechsler, P.; Smith, J. Wet but not slippery: Boundary friction in tree frog adhesive toe pads. J. R. Soc. Interface 2006, 3, 689–697. [Google Scholar] [CrossRef] [Green Version]
- Nakano, M.; Saino, T. Light and electron microscopic analyses of the high deformability of adhesive toe pads in White’s tree frog, Litoria caerulea. J. Morphol. 2016, 277, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Ba-Omar, T.A.; Downie, J.R.; Barnes, W.J.P. Development of adhesive toe-pads in the tree-frog (Phyllomedusa trinitatis). J. Zool. 2000, 250, 267–282. [Google Scholar] [CrossRef]
- Chakraborti, S.; Das, D.; De, S.K.; Nag, T.C. Structural organization of the toe pads in the amphibian Philautus annandalii (Boulenger, 1906). Acta Zool. 2014, 95, 63–72. [Google Scholar] [CrossRef]
- Nokhbatolfoghahai, M. Toe-pad morphology in White’s tree frog, Litoria caerulea (Family Hylidae). Iran. J. Sci. Technol. 2013, 37, 491–499. [Google Scholar]
- Barnes, W.J.P.; Goodwyn, P.J.P.; Nokhbatolfoghahai, M.; Gorb, S.N. Elastic modulus of tree frog adhesive toe pads. J. Comp. Physiol. A 2011, 197, 969. [Google Scholar] [CrossRef] [Green Version]
- Greenspan, S.; Longcore, J.; Calhoun, A. Host invasion by Batrachochytrium dendrobatidis: Fungal and epidermal ultrastructure in model anurans. Dis. Aquat. Organ. 2012, 100, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Berger, L.; Hyatt, A.; Speare, R.; Longcore, J. Life cycle stages of the amphibian chytrid Batrachochytrium dendrobatidis. Dis. Aquat. Organ. 2005, 68, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Pessier, A.P.; Nichols, D.K.; Longcore, J.E.; Fuller, M.S. Cutaneous chytridiomycosis in poison dart frogs (Dendrobates spp.) and White’s tree frogs (Litoria caerulea). J. Vet. Diagn. Investig. 1999, 11, 194–199. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.C.; Cramp, R.L.; Ohmer, M.E.B.; Franklin, C.E. Epidermal epidemic: Unravelling the pathogenesis of chytridiomycosis. J. Exp. Biol. 2019, 222, jeb191817. [Google Scholar] [CrossRef] [Green Version]
- Nichols, D.K.; Pessier, A.P.; Longcore, J.E. Cutaneous chytridiomycosis in amphibians: An emerging disease? In Proceedings of the AAZV and AAWV Joint Conference, Omaha, NE, USA, 17–22 October 1998; pp. 17–22. [Google Scholar]
- Reeder, N.M.M.; Pessier, A.P.; Vredenburg, V.T. A reservoir species for the emerging amphibian pathogen Batrachochytrium dendrobatidis thrives in a landscape decimated by disease. PLoS ONE 2012, 7, e33567. [Google Scholar] [CrossRef]
- Nichols, D.K.; Lamirande, E.W.; Pessier, A.P.; Longcore, J.E. Experimental transmission of cutaneous chytridiomycosis in dendrobatid frogs. J. Wildl. Dis. 2001, 37, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ohmer, M.E.B. Interactions between Amphibian Skin Sloughing and a Cutaneous Fungal Disease: Infection Progression, Immune Defence, and Phylogenetic Patterns. Ph.D. Thesis, The University of Queensland, St Lucia, QLD, Australia, 2016. [Google Scholar]
- Ohmer, M.E.B.; Alton, L.A.; Cramp, R.L. Physiology provides a window into how the multi-stressor environment contributes to amphibian declines. In Conservation Physiology; Oxford University Press: Oxford, UK, 2020; pp. 165–182. [Google Scholar]
- Endlein, T.; Barnes, W.J.P. Wet adhesion in tree and torrent frogs. In Encyclopedia of Nanotechnology; Springer: Dordrecht, The Netherlands, 2015; pp. 1–20. [Google Scholar]
- Israelachvili, J.N. Adhesion and wetting phenomena. In Intermolecular and Surface Forces; Elsevier: Amsterdam, The Netherlands, 2011; pp. 415–467. [Google Scholar]
- Langowski, J.K.A.; Rummenie, A.; Pieters, R.P.M.; Kovalev, A.; Gorb, S.N.; van Leeuwen, J.L. Estimating the maximum attachment performance of tree frogs on rough substrates. Bioinspir. Biomim. 2019, 14, 025001. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Fréchette, J. Measurement and scaling of hydrodynamic interactions in the presence of draining channels. Langmuir 2012, 28, 14703–14712. [Google Scholar] [CrossRef]
- Persson, B.N.J. Wet adhesion with application to tree frog adhesive toe pads and tires. J. Phys. Condens. Matter 2007, 19, 376110. [Google Scholar] [CrossRef]
- Bacca, M.; Booth, J.A.; Turner, K.L.; McMeeking, R.M. Load sharing in bioinspired fibrillar adhesives with backing layer interactions and interfacial misalignment. J. Mech. Phys. Solids 2016, 96, 428–444. [Google Scholar] [CrossRef]
- Barnes, W.J.P.; Pearman, J.; Platter, J. Application of peeling theory to tree frog adhesion, a biological system with biomimetic implications. E-Newslett. Sci. Technol. 2008, 1, 1–2. [Google Scholar]
- Bijma, N.N.; Gorb, S.N.; Kleinteich, T. Landing on branches in the frog Trachycephalus resinifictrix (Anura: Hylidae). J. Comp. Physiol. A 2016, 202, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Kappl, M.; Kaveh, F.; Barnes, W.J.P. Nanoscale friction and adhesion of tree frog toe pads. Bioinspir. Biomim. 2016, 11, 035003. [Google Scholar] [CrossRef] [Green Version]
- Scholz, I.; Barnes, W.J.P.; Smith, J.M.; Baumgartner, W. Ultrastructure and physical properties of an adhesive surface, the toe pad epithelium of the tree frog, Litoria caerulea White. J. Exp. Biol. 2009, 212, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Hero, J.-M.; Richards, S.; Retallick, R.; Horner, P.; Clarke, J.; Meyer, E. Litoria caerulea. The IUCN Red List of Threatened Species 2004; IUCN: Gland, Switzerland, 2004. [Google Scholar]
- Grogan, L.F.; Robert, J.; Berger, L.; Skerratt, L.F.; Scheele, B.C.; Castley, J.G.; Newell, D.A.; McCallum, H.I. Review of the amphibian immune response to chytridiomycosis, and future directions. Front. Immunol. 2018, 9, 2536. [Google Scholar] [CrossRef]
- Ramsey, J.P.; Reinert, L.K.; Harper, L.K.; Woodhams, D.C.; Rollins-Smith, L.A. Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African clawed frog, Xenopus laevis. Infect. Immun. 2010, 78, 3981–3992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollins-Smith, L.A.; Ramsey, J.P.; Pask, J.D.; Reinert, L.K.; Woodhams, D.C. Amphibian immune defenses against chytridiomycosis: Impacts of changing environments. Integr. Comp. Biol. 2011, 51, 552–562. [Google Scholar] [CrossRef]
- Langowski, J.K.A.; Singla, S.; Nyarko, A.; Schipper, H.; van den Berg, F.T.; Kaur, S.; Astley, H.C.; Gussekloo, S.W.S.; Dhinojwala, A.; van Leeuwen, J.L. Comparative and functional analysis of the digital mucus glands and secretions of tree frogs. Front. Zool. 2019, 16, 19. [Google Scholar] [CrossRef] [Green Version]
- Schöning-Langowski, J.K.A. Getting a Grip on Tree Frog Attachment: Structures, Mechanisms, and Biomimetic Potential. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2019. [Google Scholar]
- Campbell, C.R.; Voyles, J.; Cook, D.I.; Dinudom, A. Frog skin epithelium: Electrolyte transport and chytridiomycosis. Int. J. Biochem. Cell Biol. 2012, 44, 431–434. [Google Scholar] [CrossRef] [Green Version]
- AmphibiaWeb. 2021. University of California, Berkeley, USA. Available online: https://amphibiaweb.org (accessed on 8 June 2021).
- Blaustein, A.R.; Walls, S.C.; Bancroft, B.A.; Lawler, J.J.; Searle, C.L.; Gervasi, S.S. Direct and indirect effects of climate change on amphibian populations. Diversity 2010, 2, 281–313. [Google Scholar] [CrossRef]
- Rödder, D.; Kielgast, J.; Bielby, J.; Schmidtlein, S.; Bosch, J.; Garner, T.W.; Veith, M.; Walker, S.; Fisher, M.; Lötters, S. Global amphibian extinction risk assessment for the panzootic chytrid fungus. Diversity 2009, 1, 52–66. [Google Scholar] [CrossRef]
- Hero, J.-M.; Williams, S.E.; Magnusson, W.E. Ecological traits of declining amphibians in upland areas of eastern Australia. J. Zool. 2005, 267, 221. [Google Scholar] [CrossRef]
- Zumbado-Ulate, H.; Nelson, K.N.; García-Rodríguez, A.; Chaves, G.; Arias, E.; Bolaños, F.; Whitfield, S.M.; Searle, C.L. Endemic infection of Batrachochytrium dendrobatidis in Costa Rica: Implications for amphibian conservation at regional and species level. Diversity 2019, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Endlein, T.; Barnes, W.J.P.; Samuel, D.S.; Crawford, N.A.; Biaw, A.B.; Grafe, U. Sticking under wet conditions: The remarkable attachment abilities of the torrent frog, Staurois guttatus. PLoS ONE 2013, 8, e73810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conlon, J.M.; Mechkarska, M. Host-defense peptides with therapeutic potential from skin secretions of frogs from the family Pipidae. Pharmaceuticals 2014, 7, 58–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musale, V.; Casciaro, B.; Mangoni, M.L.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; Conlon, J.M. Assessment of the potential of temporin peptides from the frog Rana temporaria (Ranidae) as anti-diabetic agents. J. Pept. Sci. 2018, 24, e3065. [Google Scholar] [CrossRef] [PubMed]
- Cerullo, A.R.; Lai, T.Y.; Allam, B.; Baer, A.; Barnes, W.J.P.; Barrientos, Z.; Deheyn, D.D.; Fudge, D.S.; Gould, J.; Harrington, M.J.; et al. Comparative animal mucomics: Inspiration for functional materials from ubiquitous and understudied biopolymers. ACS Biomater. Sci. Eng. 2020, 6, 5377–5398. [Google Scholar] [CrossRef]
- Langowski, J.K.A.; Sharma, P.; Shoushtari, A.L. In the soft grip of nature. Sci. Robot. 2020, 5, eabd9120. [Google Scholar] [CrossRef]



| Ref. | Species | Skin Location | Method | Infection Severity | Observed Skin Alterations |
|---|---|---|---|---|---|
| [43] | Litoria caerulea | Abdominal pelvic patch | Histology | Varying (approximated by zoospore load) | - Cutaneous erythema, histological lesions and thinning of the skin |
| [8] | L. caerulea | Digital | Histology | High* | - Stratum corneum thickened from 2–5 µm to 60 µm - Mature sporangia diameter = 12–20 µm |
| [9] | L. caerulea | Ventral skin | Histology | High* | - Hyperplasia thickens epidermis from 25–60 µm (4–7 layers) to up to 125 µm (13 layers) |
| [44] | L. caerulea | Ventral abdomen, pelvis, and hindlimbs | Histology | High* | - Cutaneous lesions characterized by hyperplasia and hyperkeratosis |
| [41] | L. gracilenta | Toe clip | TEM | Varying (one animal with mild infection, one died from infection) | - Up to 4 extra keratinized skin layers in stratum corneum (mild infection) - “Fibrillar zone” (2.5 µm wide) around sporangia |
| [41] | L. lesueuri | Interdigital | SEM | Unknown | - Bulging epidermal cells with discharge tubes covering ca. 10% of the cell surface - Up to 3 sporangia in an epidermal cell - Sporangia diameter up to 40 µm |
| [45] | Pseudacris regilla | Ventral pelvic patch, foot webbing | Histology | Varying (five animals with non-lethal infection, one died from infection) | - Hyperkeratosis and hyperplasia across the whole skin (lethally affected individual) or in discrete patches (non-lethally affected individuals) - Cutaneous lesions with high density of Bd-sporangia |
| [40] | Lithobates catesbeianus | Interdigital | EM | Low (study of the infection process) | - Clear keratin-free zones of host cytoplasm around zoosporangium - Premature keratinization of 2–3 outer cell layers |
| [46] | Dendrobates spec. | Various | Histology | High * | - Hyperkeratosis, hyperplasia, and/or hypertrophy of nonkeratinized epidermal cells |
| [42] | Dendrobates azureus | Various | Histology | High * | - Germination tubes extend towards the skin surface |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieuwboer, L.; van Leeuwen, J.L.; Martel, A.; Pasmans, F.; Spitzen-van der Sluijs, A.; Langowski, J.K.A. Does Chytridiomycosis Affect Tree Frog Attachment? Diversity 2021, 13, 262. https://doi.org/10.3390/d13060262
Nieuwboer L, van Leeuwen JL, Martel A, Pasmans F, Spitzen-van der Sluijs A, Langowski JKA. Does Chytridiomycosis Affect Tree Frog Attachment? Diversity. 2021; 13(6):262. https://doi.org/10.3390/d13060262
Chicago/Turabian StyleNieuwboer, Lisa, Johan L. van Leeuwen, An Martel, Frank Pasmans, Annemarieke Spitzen-van der Sluijs, and Julian K. A. Langowski. 2021. "Does Chytridiomycosis Affect Tree Frog Attachment?" Diversity 13, no. 6: 262. https://doi.org/10.3390/d13060262
APA StyleNieuwboer, L., van Leeuwen, J. L., Martel, A., Pasmans, F., Spitzen-van der Sluijs, A., & Langowski, J. K. A. (2021). Does Chytridiomycosis Affect Tree Frog Attachment? Diversity, 13(6), 262. https://doi.org/10.3390/d13060262