Effects of Long-Term Habitat Protection on Montane Small Mammals: Are Sorex araneus and S. minutus More Sensitive Than Previously Considered?
Abstract
:1. Introduction
2. Materials and Methods
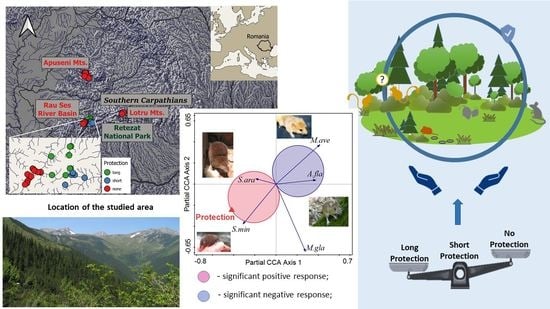
2.1. Description of the Study Areas
2.2. Protection Status and Habitat Variables
2.3. Small Mammal Trapping
2.4. Data Analysis
3. Results
3.1. Trapping Results
3.2. Effect of Long-Term Protection on Montane Small Mammals
3.3. Responses of Small Mammal Species Composition
4. Discussion
4.1. Effects of Habitat Protection Status
4.2. Other Patterns
4.3. Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayyad, M.A. Case studies in the conservation of biodiversity: Degradation and threats. J. Arid. Environ. 2003, 54, 165–182. [Google Scholar] [CrossRef] [Green Version]
- Hermy, M.; Van der Veken, S.; Van Calster, H.; Plue, J. Forest ecosystem assessment, changes in biodiversity and climate change in a densely populated region (Flanders, Belgium). Plant Biosyst. 2008, 142, 623–629. [Google Scholar] [CrossRef]
- Zambrano-Monserrate, M.A.; Carvajal-Lara, C.; Urgilés-Sanchez, R.; Ruano, M.A. Deforestation as an indicator of environmental degradation: Analysis of five European countries. Ecol. Indic. 2018, 90, 1–8. [Google Scholar] [CrossRef]
- Pendrill, F.; Persson, U.M.; Godar, J.; Kastner, T.; Moran, D.; Schmidt, S.; Wood, R. Agricultural and forestry trade drives large share of tropical deforestation emissions. Glob. Environ. Change 2019, 56, 1–10. [Google Scholar] [CrossRef]
- O’Neill, C.; Lim, F.K.; Edwards, D.P.; Osborne, C.P. Forest regeneration on European sheep pasture is an economically viable climate change mitigation strategy. Environ. Res. Lett. 2020, 15, 104090. [Google Scholar] [CrossRef]
- Veen, P.; Fanta, J.; Raev, I.; Biriş, I.A.; de Smidt, J.; Maes, B. Virgin forests in Romania and Bulgaria: Results of two national inventory projects and their implications for protection. Biodivers. Conserv. 2010, 19, 1805–1819. [Google Scholar] [CrossRef]
- Parviainen, J. Virgin and natural forests in the temperate zone of Europe. For. Snow Landsc. Res. 2005, 79, 9–18. [Google Scholar]
- Nagendra, H.; Lucas, R.; Honrado, J.P.; Jongman, R.H.G.; Tarantino, C.; Adamo, M.; Mairota, P. Remote sensing for conservation monitoring: Assessing protected areas, habitat extent, habitat condition, species diversity, and threats. Ecol. Indic. 2012, 33, 45–59. [Google Scholar] [CrossRef]
- Watson, J.E.M.; Dudley, N.; Segan, D.B.; Hockings, M. The performance and potential of protected areas. Nature 2014, 515, 67–73. [Google Scholar] [CrossRef]
- Gray, C.L.; Hill, S.L.L.; Newbold, T.; Hudson, L.N.; Börger, L.; Contu, S.; Hoskins, A.J.; Ferrier, S.; Purvis, A.; Scharlemann, J.P.W. Local biodiversity is higher inside than outside terrestrial protected areas worldwide. Nat. Commun. 2016, 7, 12306. [Google Scholar] [CrossRef] [Green Version]
- Fuentes-Montemayor, E.; Ferryman, M.; Watts, K.; Macgregor, N.A.; Hambly, N.; Brennan, S.; Park, K.J. Small mammal responses to long-term large-scale woodland creation: The influence of local and landscape-level attributes. Ecol. Appl. 2020, 30, e02028. [Google Scholar] [CrossRef] [Green Version]
- Davies, G.T.O.; Kirkpatrick, J.B.; Cameron, E.Z.; Carver, S.; Johnson, C.N. Ecosystem engineering by digging mammals: Effects on soil fertility and condition in Tasmanian temperate woodland. R. Soc. Open Sci. 2018, 6, 180621. [Google Scholar] [CrossRef] [Green Version]
- Wijnhoven, S.; Thonon, I.; Van Der Velde, G.; Leuven, R.; Zorn, M.; Eijsackers, H.; Smits, T. The impact of bioturbation by small mammals on heavy metal redistribution in an embanked floodplain of the river Rhine. Water Air Soil Pollut. 2006, 177, 183–210. [Google Scholar] [CrossRef] [Green Version]
- Lagendijk, D.D.G.; Howison, R.A.; Esselink, P.; Smit, C. Grazing as a conservation management tool: Responses of voles to grazer species and densities. Basic Appl. Ecol. 2019, 34, 36–45. [Google Scholar] [CrossRef]
- Perea, R.; San Miguel, A.; Gil, L. Acorn dispersal by rodents: The importance of re-dispersal and distance to shelter. Basic Appl. Ecol. 2011, 12, 432–439. [Google Scholar] [CrossRef]
- Wells, K.; Corlett, R.T.; Lakim, M.B.; Kalko, E.K.V.; Pfeiffer, M. Seed consumption by small mammals from Borneo. J. Trop. Ecol. 2009, 25, 555–558. [Google Scholar] [CrossRef] [Green Version]
- Campos, C.M.; Campos, V.E.; Giannoni, S.M.; Rodriguez, D.; Albanese, S.; Cona, M.I. Role of small rodents in the seed dispersal process: Microcavia australis consuming Prosopis flexuosa fruits. Austral. Ecol. 2017, 42, 113–119. [Google Scholar] [CrossRef]
- Stephens, R.B.; Rowe, R.J. The underappreciated role of rodent generalists in fungal spore dispersal networks. Ecology 2020, 101, e02972. [Google Scholar] [CrossRef]
- Schickmann, S.; Urban, A.; Kräutler, K.; Nopp-Mayr, U.; Hackländer, K. The interrelationship of mycophagous small mammals and ectomycorrhizal fungi in primeval, disturbed and managed Central European mountainous forests. Oecologia 2012, 170, 395–409. [Google Scholar] [CrossRef] [Green Version]
- Paz, C.; Öpik, M.; Bulascoschi, L.; Bueno, C.G.; Galetti, M. Dispersal of arbuscular mycorrhizal fungi: Evidence and insights for ecological studies. Microb. Ecol. 2021, 81, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Churchfield, S.; Hollier, J.; Brown, V.K. The effects of small mammal predators on grassland invertebrates, investigated by field exclosure experiment. Oikos 1991, 60, 283–290. [Google Scholar] [CrossRef]
- Lukyanova, L.E.; Ukhova, N.L.; Ukhova, O.V.; Gorodilova, Y.V. Common shrew (Sorex araneus, Eulipotyphla) population and the food supply of its habitats in ecologically contrasting environments. Russ. J. Ecol. 2021, 52, 316–328. [Google Scholar] [CrossRef]
- Simard, J.R.; Fryxell, J.M. Effects of selective logging on terrestrial small mammals and arthropods. Can. J. Zool. 2003, 81, 1318–1326. [Google Scholar] [CrossRef]
- Dickman, C.R.; Doncaster, C.P. The ecology of small mammals in urban habitats. I. Populations in a patchy environment. J. Anim. Ecol. 1987, 56, 629–640. [Google Scholar] [CrossRef]
- Romanowski, J.; Lesiński, G.; Bardzińska, M. Small mammals of the suburban areas of Warsaw in the diet of the tawny owl Strix aluco. Stud. Ecol. Bioeth. 2020, 18, 349–354. [Google Scholar] [CrossRef]
- Geduhn, A.; Esther, A.; Schenke, D.; Mattes, H.; Jacob, J. Spatial and temporal exposure patterns in non-target small mammals during brodifacoum rat control. Sci. Total Environ. 2014, 496, 328–338. [Google Scholar] [CrossRef]
- Olson, L.E.; Squires, J.R.; Oakleaf, R.J.; Wallace, Z.P.; Kennedy, P.L. Predicting above-ground density and distribution of small mammal prey species at large spatial scales. PLoS ONE 2017, 12, e0177165. [Google Scholar] [CrossRef]
- Pacheco, V.; Salas, E.; Barriga, C.; Rengifo, E. Small mammal diversity in disturbed and undisturbed montane forest in the area of influence of the PERU LNG pipeline, Apurímac River watershed, Ayacucho, Peru. In Monitoring Biodiversity, Lessons from a Trans-Andean Megaproject; Alonso, A., Dallmeier, F., Servat, G.P., Eds.; Smithsonian Institution Scholary Press: Washington, DC, USA, 2013; pp. 90–100. [Google Scholar]
- Krojerová-Prokešová, J.; Heroldová, M.; Barančeková, M.; Homolka, M. Patterns of vole gnawing on saplings in managed clearings in Central European forests. For. Ecol. Manag. 2017, 408, 137–147. [Google Scholar] [CrossRef]
- Henttonen, H.; Haukisalmi, V.; Kaikusalo, A.; Korpimäki, E.; Norrdahl, K.; Skarén, U.A.P. Long-term population dynamics of the common shrew Sorex araneus in Finland. Ann. Zool. Fenn. 1989, 26, 349–355. [Google Scholar]
- Michał, B.; Rafał, Z. Responses of small mammals to clear-cutting in temperate and boreal forests of Europe: A meta-analysis and review. Eur. J. For. Res. 2014, 133, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ivanter, E.V.; Kurhinen, J.P. Effect of anthropogenic transformation of forest landscapes on populations of small insectivores in eastern Fennoscandia. Russ. J. Ecol. 2015, 46, 252–259. [Google Scholar] [CrossRef]
- Bryja, J.; Rehak, Z. Community of small terrestrial mammals (Insectivora, Rodentia) in dominant habitats of the Protected Landscape Area of Poodří. Flora Zool. 1998, 47, 249–260. [Google Scholar]
- Lesiński, G.; Gryz, J.B. How protecting a suburban forest as a natural reserve affected small mammal communities. Urban Ecosyst. 2012, 15, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Men, X.; Guo, X.; Dong, W.; Ding, N.; Qian, T. Influence of human disturbance to the small mammal communities in the forests. Open J. For. 2015, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Caro, T.M. Factors affecting the small mammal community inside and outside Katavi National Park, Tanzania. BioTropica 2002, 34, 310–318. [Google Scholar] [CrossRef]
- Konečný, A.; Koubek, P.; Bryja, J. Indications of higher diversity and abundance of small rodents in human-influenced Sudanian savannah than in the Niokolo Koba National Park (Senegal). Afr. J. Ecol. 2010, 48, 718–726. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2020. Available online: https://qgis.org/en/site/forusers/index.html#download (accessed on 15 September 2020).
- Patterson, T.; Kelso, N.V. Natural Earth. Free Vector and Raster Map Data. 2020. Available online: https://www.naturalearthdata.com (accessed on 19 September 2020).
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004; p. 256. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-6. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 17 January 2020).
- R Core Development Team. R: A Language and Environment for Statistical Computing, version 3.6.1; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: http://www.r-project.org (accessed on 14 November 2019).
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: iNterpolation and EXTrapolation for Species Diversity. R Package Version 2.0.20. 2020. Available online: http://chao.stat.nthu.edu.tw/wordpress/software-download/ (accessed on 26 December 2021).
- Benedek, A.M.; Sîrbu, I. Dynamics of small-mammal communities along an elevational gradient. Can. J. Zool. 2019, 97, 312–318. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Nakagawa, S.; Johnson, P.C.D.; Schielzeth, H. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 2017, 14, 134. [Google Scholar] [CrossRef] [Green Version]
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.43.15. 2019. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 6 March 2020).
- Ter Braak, C.J.F.; Šmilauer, P. Canoco for Windows Version 5.10; Biometris—Plant Research International: Wageningen, The Netherlands, 2018. [Google Scholar]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using Canoco 5, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Lešo, P.; Kropil, R. Is the common shrew (Sorex araneus) really a common forest species? Rend. Fis. Acc. Lincei 2017, 28, 183–189. [Google Scholar] [CrossRef]
- Nicolas, V.; Colyn, M. Relative efficiency of three types of small mammal traps in an African rainforest. Belg. J. Zool. 2006, 136, 107–111. [Google Scholar]
- Do, R.; Shonfield, J.; McAdam, A.G. Reducing accidental shrew mortality associated with small-mammal livetrapping II: A field experiment with bait supplementation. J. Mammal. 2013, 94, 754–760. [Google Scholar] [CrossRef] [Green Version]
- Zárybnická, M.; Riegert, J.; Bejček, V.; Sedláček, F.; Šťastný, K.; Šindelář, J.; Heroldová, M.; Vilímová, J.; Zima, J. Long-term changes of small mammal communities in heterogenous landscapes of Central Europe. Eur. J. Wildl. Res. 2017, 63, 89. [Google Scholar] [CrossRef]
- Baláz, I.; Ambros, M. Relationship of shrews (Sorex sp.) to forest biotopes in Western Carpathians. Ekológia 2005, 24, 254–262. [Google Scholar]
- Klenovšek, T.; Novak, T.; Čas, M.; Trilar, T.; Janžekovič, F. Feeding ecology of three sympatric Sorex shrew species in montane forest Slovenia. Folia Zool. 2013, 62, 193–199. [Google Scholar] [CrossRef]
- Bright, P.; Morris, P.; Mitchell-Jones, T. The Dormouse Conservation Handbook, 2nd ed.; English Nature: Peterborough, UK, 2006; p. 13. [Google Scholar]
- Bailey, S. Increasing connectivity in fragmented landscapes: An investigation of evidence for biodiversity gain in woodlands. For. Ecol. Manag. 2007, 238, 7–23. [Google Scholar] [CrossRef]
- Goodwin, C.E.; Suggitt, A.J.; Bennie, J.; Silk, M.J.; Duffy, J.P.; Al-Fulaij, N.; Bailey, S.; Hodgson, D.J.; McDonald, R.A. Climate, landscape, habitat, and woodland management associations with hazel dormouse Muscardinus avellanarius population status. Mamm. Rev. 2018, 48, 209–223. [Google Scholar] [CrossRef] [Green Version]
- Williams, S.E.; Marsh, H.; Winter, J. Spatial scale, species diversity, and habitat structure: Small mammals in australian tropical rain forest. Ecology 2002, 83, 1317–1329. [Google Scholar] [CrossRef]
- Rowe, R.J. Environmental and geometric drivers of small mammal diversity along elevational gradients in Utah. Ecography 2009, 32, 411–422. [Google Scholar] [CrossRef]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Pik, A.J.; Dangerfield, J.M.; Bramble, R.A.; Angus, C.; Nipperess, D.A. The use of invertebrates to detect small-scale habitat heterogeneity and its application to restoration practices. Environ. Monit. Assess. 2002, 75, 179–199. [Google Scholar] [CrossRef]
- Marsh, A.C.W.; Poulton, S.; Harris, S. The yellow-necked mouse Apodemus flavicollis in Britain: Status and analysis of factors affecting distribution. Mamm. Rev. 2001, 31, 203–227. [Google Scholar] [CrossRef]
- Benedek, A.M.; Sîrbu, I.; Lazăr, A. Responses of small mammals to habitat characteristics in Southern Carpathian forests. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ylönen, H.; Altner, H.J.; Stubbe, M. Seasonal dynamics of small mammals in an isolated woodlot and its agricultural surroundings. Ann. Zool. Fenn. 1991, 28, 7–14. [Google Scholar]
- Heldstab, S.A. Latitude, life history and sexual size dimorphism correlate with reproductive seasonality in rodents. Mamm. Rev. 2021, 52, 256–271. [Google Scholar] [CrossRef]
- Juškaitis, R. Hazel dormice (Muscardinus avellanarius) in a regenerating clearing: The effects of clear-felling and regrowth thinning on long-term abundance dynamics. Eur. J. Wild. Res. 2020, 66, 48. [Google Scholar] [CrossRef]
- Ramakers, J.J.C.; Dorenbosch, M.; Foppen, R.P.B. Surviving on the edge: A conservation-oriented habitat analysis and forest edge manipulation for the hazel dormouse in the Netherlands. Eur. J. Wild. Res. 2014, 60, 927–993. [Google Scholar] [CrossRef]




| Species | Not Protected | Short Protection | Long Protection | Total |
|---|---|---|---|---|
| Apodemus agrarius | 1 | 0 | 0 | 1 |
| Apodemus flavicollis | 68 | 26 | 18 | 112 |
| Chionomys nivalis | 0 | 1 | 0 | 1 |
| Glis glis | 1 | 0 | 0 | 1 |
| Microtus agrestis | 1 | 4 | 0 | 5 |
| Muscardinus avellanarius | 7 | 2 | 0 | 9 |
| Myodes glareolus | 47 | 31 | 40 | 118 |
| Microtus subterraneus | 0 | 0 | 1 | 1 |
| Neomys anomalus | 0 | 1 | 0 | 1 |
| Neomys fodiens | 2 | 0 | 1 | 3 |
| Sorex alpinus | 2 | 2 | 1 | 5 |
| Sorex araneus | 19 | 27 | 35 | 81 |
| Sorex minutus | 0 | 2 | 3 | 5 |
| Total individuals | 148 | 96 | 99 | 343 |
| Overall capture index (individuals/100 TN) | 7.45 | 10.4 | 13.21 | 9.37 |
| Captured species | 9 | 9 | 7 | 13 |
| Transects (visits) | 34 | 24 | 17 | 75 |
| Empty transects (visits) | 9 | 1 | 3 | 13 |
| Trapping effort (TN) | 1985 | 923 | 749 | 3657 |
| Elevational range of trapping sites (m) | 840–1650 | 920–1550 | 820–1750 | 820–1750 |
| Model (Predictors) | Coefficient Estimate | Standard Error | Chi-Square LR Test | p | Conditional R2 | Marginal R2 |
|---|---|---|---|---|---|---|
| Total abundance * (negative binomial) | 0.664 | 0.204 | ||||
| Intercept | −0.078 | 0.655 | ||||
| Elev | −1.151 | 0.398 | 7.598 | 0.005 | ||
| Prot | 0.438 | 0.123 | 11.618 | <0.001 | ||
| Month | 0.276 | 0.089 | 8.653 | 0.003 | ||
| Hab.heterog | 0.637 | 0.22 | 7.950 | 0.004 | ||
| A. flavicollis * (negative binomial) | 0.772 | 0.122 | ||||
| Intercept | 0.579 | 1.384 | ||||
| Elev | −2.593 | 0.828 | 11.518 | <0.001 | ||
| Hab.heterog | 0.995 | 0.453 | 4.325 | 0.037 | ||
| M. glareolus * (negative binomial) | 0.214 | 0.067 | ||||
| Intercept | −0.985 | 0.481 | ||||
| Prot | 0.468 | 0.199 | 5.989 | 0.014 | ||
| Elev low | 0.295 | 0.429 | 5.685 | 0.058 | ||
| Elev medium | 0.891 | 0.387 | ||||
| S. araneus (negative binomial) | 0.408 | |||||
| Intercept | −3.729 | 0.634 | ||||
| Month | 0.855 | 0.180 | 23.665 | <0.001 | ||
| Prot | 0.53 | 0.2 | 6.076 | 0.013 | ||
| S. minutus (binomial) | 0.167 | |||||
| Intercept | −4.997 | 1.216 | ||||
| Prot | 1.586 | 0.722 | 6.588 | 0.01 | ||
| M. avellanarius (binomial) | 0.079 | |||||
| Intercept | −2.091 | 0.44 | ||||
| Prot | −1.138 | 0.704 | 3.764 | 0.055 | ||
| S * (Gaussian) | 0.299 | 0.069 | ||||
| Intercept | 1.12 | 0.407 | ||||
| Month | 0.251 | 0.109 | 5.173 | 0.022 | ||
| Chao (Gaussian) | 0.036 | |||||
| Intercept | 1.246 | 0.413 | ||||
| Month | 0.266 | 0.137 | 3.77 | 0.052 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedek, A.M.; Lazăr, A.; Cic, N.V.; Cocîrlea, M.D.; Sîrbu, I. Effects of Long-Term Habitat Protection on Montane Small Mammals: Are Sorex araneus and S. minutus More Sensitive Than Previously Considered? Diversity 2022, 14, 38. https://doi.org/10.3390/d14010038
Benedek AM, Lazăr A, Cic NV, Cocîrlea MD, Sîrbu I. Effects of Long-Term Habitat Protection on Montane Small Mammals: Are Sorex araneus and S. minutus More Sensitive Than Previously Considered? Diversity. 2022; 14(1):38. https://doi.org/10.3390/d14010038
Chicago/Turabian StyleBenedek, Ana Maria, Anamaria Lazăr, Niculina Viorica Cic, Maria Denisa Cocîrlea, and Ioan Sîrbu. 2022. "Effects of Long-Term Habitat Protection on Montane Small Mammals: Are Sorex araneus and S. minutus More Sensitive Than Previously Considered?" Diversity 14, no. 1: 38. https://doi.org/10.3390/d14010038
APA StyleBenedek, A. M., Lazăr, A., Cic, N. V., Cocîrlea, M. D., & Sîrbu, I. (2022). Effects of Long-Term Habitat Protection on Montane Small Mammals: Are Sorex araneus and S. minutus More Sensitive Than Previously Considered? Diversity, 14(1), 38. https://doi.org/10.3390/d14010038