Trophic Niche Dynamics and Diet Partitioning of King Crab Lithodes santolla in Chile’s Sub-Antarctic Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling
2.3. Stomach Content Analysis (SCA)
2.4. Stable Isotope Analysis (SIA)
2.5. Data Analysis
3. Results
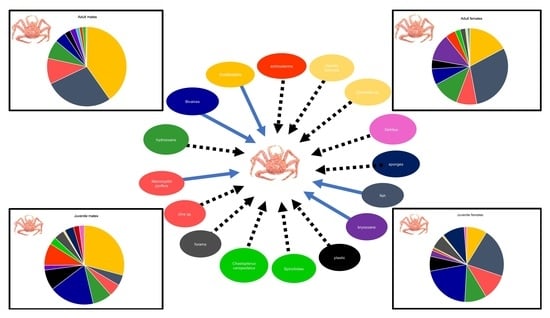
3.1. Stomach Content
3.2. King Crab Stable Isotope Composition and Potential Basal Carbon Sources
3.3. Isotopic Niche and Overlap
3.4. Trophic Position and Ontogenetic Shift
3.5. Relationship between Body Size, Body Mass, TP and Isotopic Values of Lithodes santolla
3.6. Community Niche Width and Food Web Length
4. Discussion
4.1. Diet Composition of Lithodes santolla
4.2. Contribution of Kelp Carbon as the Major Source to Lithodes santolla
4.3. Intraspecific Niche Variation and Overlap of Lithodes santolla
4.4. Trophic Position of Lithodes santolla
4.5. Community Niche Width
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pollack, G.; Berghöfer, A.; Berghöfer, U. Fishing for social realities—Challenges to sustainable fisheries management in the Cape Horn Biosphere Reserve. Mar. Policy 2008, 32, 233–242. [Google Scholar] [CrossRef]
- Stuardo, J.; Solis, I. Biometría y observaciones generales sobre la biología de Lithodes antarcticus Jacquinot. Gay. Zool. 1963, 11, 1–52. [Google Scholar]
- Campodónico, I.; Hernández, M.; Riveros, E. Investigación, manejo y control de las pesquerías de centolla y centollón de la XII región. Informe Consolidado: Recurso centollón. Punta Arenas, Chile. 1983. Available online: http://www.bibliotecadigital.umag.cl (accessed on 14 January 2019).
- Guzmán, L.; Ríos, C. Investigación, manejo y control de las pesquerías de centolla y centollón de la Xlla región 1979–1983 informe Consolidado: Recurso centolla (Lithodes antarcticus Jacquinot). Punta Arenas, Chile. 1985. Available online: http://www.bibliotecadigital.umag.cl (accessed on 26 January 2019).
- Vinuesa, J. Sistema reproductor, ciclo y madurez gonadal de la centolla (Lithodes antarcticus) del canal Beagle. Contrib. INIDEP 1984, 441, 73–95. [Google Scholar]
- Cañete, J.I.; Cárdenas, C.; Oyarzún, S.; Plana, J.; Palacios, M.; Santana, M. Pseudione tuberculata Richardson, 1904 (Isopoda: Bopyridae): A parasite of juveniles of the king crab Lithodes santolla (Molina, 1782) (Anomura: Lithodidae) in the Magellan Strait, Chile. Rev. Biol. Mar. Oceanogr. 2008, 43, 265–274. [Google Scholar] [CrossRef]
- Lovrich, G.; Tapella, F. Southern king crabs. In King Crabs of the World: Biology and Fisheries Management; Stevens, B.G., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 405–449. ISBN 978-143-985-541-6. [Google Scholar]
- Cárdenas, C.; Cañete, J.I.; Oyarzún, S.; Mansilla, A. Podding of juvenile king crabs Lithodes santolla (Molina, 1782) (Crustacea) in association with holdfasts of Macrocystis pyrifera (Linnaeus) C. Agardh, 1980. Investig. Mar. 2007, 35, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Sotelano, P.; Lovrich, G.; Romero, C.; Tapella, F. Cannibalism during intermolt period in early stages of the Southern King Crab Lithodes santolla (Molina 1872): Effect of stage and predator–prey proportions. J. Exp. Mar. Biol. Ecol. 2012, 411, 52–58. [Google Scholar] [CrossRef]
- Garay-Flühmann, R.; Garay-Narváez, L.; Montenegro, C.; Galán, A.; Olguín, A.; Andrade, C.; Pinilla, E.; Vidal, G.; Aedo, G.; Contreras, H.; et al. Modelamiento ecológico conceptual y cualitativo para recursos centolla (Lithodes santolla) y centollón (Paralomis granulosa) Región de Magallanes y la Antártica Chilena y de jaiba marmola (Metacarcinus edwardsii), Chiloé, Región de Los Lagos. IFOP: Valparaíso, Chile, 2019. Available online: http://www.ifop.cl/enfoque-ecosistemico/wp-content/uploads/sites/19/2019/07/INFORME_T%C3%89CNICO_10_-_MOD.CUALIT._DIMENSI%C3%93N_ECOL%C3%93GICA_MAGALLANES_-_CHILO%C3%89.pdf (accessed on 10 January 2020).
- Botsford, L.; Castilla, J.C.; Peterson, C. The management of fisheries and marine ecosystems. Science 1997, 277, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Hubans, B.; Chouvelon, T.; Begout, M.L.; Biais, G.; Bustamante, P.; Ducci, L.; Mornet, F.; Boiron, A.; Coupeau, Y.; Splitz, J. Trophic ecology of commercial-size meagre, Argyrosomus regius, in the Bay of Biscay (NE Atlantic). Aquat. Living Resour. 2017, 30, 9. [Google Scholar] [CrossRef] [Green Version]
- SERNAPESCA Home Page. Available online: http://www.sernapesca.cl/informes/estadisticas (accessed on 30 November 2020).
- Vinuesa, J.; Varisco, M.; Balzi, P. Feeding strategy of early juvenile stages of the southern king crab Lithodes santolla in the San Jorge Gulf, Argentina. Rev. Biol. Mar. Oceanogr. 2013, 48, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Comoglio, L.; Amin, O. Dieta natural de la centolla patagónica Lithodes santolla (Lithodidae) en el Canal Beagle, Tierra del Fuego, Argentina. Biol. Pesq. 1996, 25, 51–57. [Google Scholar]
- Balzi, P. Los hábitos alimenticios de la centolla Lithodes santolla (Molina) del Golfo San Jorge. Nat. Patagónica Cienc. Biol. 1997, 5, 67–87. [Google Scholar]
- Lovrich, G. Lithodidae. In Los Invertebrados del mar Argentino; Calcagno, J.A., Ed.; Vázquez Mazzini Editores: Buenos Aires, Argentina, 2014; pp. 243–263. ISBN 978-987-3781-02-5. [Google Scholar]
- Tapella, F.; Lovrich, G.A. Asentamiento de estadios tempranos de las centollas Lithodes santolla y Paralomis granulosa (Decapoda: Lithodidae) en colectores artificiales pasivos en el Canal Beagle. Argentina. Investig. Mar. 2006, 34, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Pardo, L.M.; Andrade, C.; Zenteno-Devaud, L.; Garrido, B.; Rivera, C. Trophic Ecology of Juvenile Southern King Crab Associated with Kelp Forest: Evidence of Cannibalism. Diversity 2021, 13, 556. [Google Scholar] [CrossRef]
- Figueroa-Muñoz, G.; Retamal, M.; De Los Ríos, P.R.; Esse, C.; Pérez-Schultheiss, J.; Vega-Aguayo, R.; Boyero, L.; Correa-Araneda, F. Scavenging crustacean fauna in the Chilean Patagonian Sea. Sci. Rep. 2020, 10, 5940. [Google Scholar] [CrossRef] [Green Version]
- Vinuesa, J. Distribución de crustáceos decápodos y estomatópodos del golfo San Jorge, Argentina. Rev. Biol. Mar. Oceanogr. 2005, 40, 7–21. [Google Scholar] [CrossRef]
- Svanbäck, R.; Bolnick, D. Intraspecific competition affects the strength of individual specialization: An optimal diet theory method. Evol. Ecol. Res. 2005, 7, 993–1012. [Google Scholar]
- Chase, J.; Leibold, M. Ecological Niches: Linking Classical and Contemporary Approaches; The University of Chicago Press: Chicago, IL, USA, 2003; p. 221. ISBN 0-226-10180-0. [Google Scholar]
- Newsome, S.D.; Martinez del Rio, C.; Bearhop, S.; Phillips, D.L. A niche for isotopic ecology. Front. Ecol. Environ. 2007, 5, 429–436. [Google Scholar] [CrossRef]
- Squires, H.; Dawe, E. Stomach contents of snow crab (Chionoecetes opilio, Decapoda, Brachyura) from the Northeast Newfoundland Shelf. J. Northwest Atl. Fish. Sci. 2003, 32, 27–38. [Google Scholar] [CrossRef]
- Fuhrmann, M.; Pedersen, T.; Nilssen, E. Trophic niche of the invasive red king crab Paralithodes camtschaticus in a benthic food web. Mar. Ecol. Prog. Ser. 2017, 565, 113–129. [Google Scholar] [CrossRef]
- Falk-Petersen, J.; Renaud, P.; Anisimova, N. Establishment and ecosystem effects of the alien invasive red king crab (Paralithodes camtschaticus) in the Barents Sea—A review. J. Mar. Sci. 2011, 68, 479–488. [Google Scholar] [CrossRef] [Green Version]
- Michener, R.; Schell, D. Stable isotope ratios as tracers in marine aquatic food webs. In Stable Isotopes in Ecology and Environmental Science; Michener, R., Lajtha, K., Eds.; Blackwell Scientific Publications: Oxford, UK, 1994; pp. 138–157. ISBN 978-140-512-680-9. [Google Scholar]
- Bearhop, S.; Adams, C.E.; Waldron, S.; Fuller, R.A.; MacLeod, H. Determining trophic niche width: A novel approach using stable isotope analysis. J. Anim. Ecol. 2004, 73, 1007–1012. [Google Scholar] [CrossRef] [Green Version]
- Layman, C.A.; Arrington, D.A.; Montaña, C.G.; Post, D.M. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 2007, 88, 4248. [Google Scholar] [CrossRef]
- Swanson, H.; Lysy, M.; Power, M.; Stasko, A.; Johnson, J.; Reist, J. A new probabilistic method for quantifying n-dimensional ecological niches and niche overlap. Ecology 2015, 96, 318–324. [Google Scholar] [CrossRef]
- Divine, L.; Bluhm, B.; Mueter, F.; Iken, K. Diet analysis of Alaska Arctic snow crabs (Chionoecetes opilio) using stomach contents and δ¹³C and δ¹⁵N stable isotopes. Deep-Sea Res. II Top. Stud. Oceanogr. 2017, 135, 124–136. [Google Scholar] [CrossRef] [Green Version]
- Friedlander, A.; Ballesteros, E.; Bell, T.; Giddens, J.; Henning, B.; Hüne, M.; Muñoz, A.; Salinas de León, P.; Sala, E. Marine biodiversity at the end of the world: Cape Horn and Diego Ramírez islands. PLoS ONE 2018, 13, e0189930. [Google Scholar] [CrossRef] [Green Version]
- Valdenegro, A.; Silva, N. Caracterización oceanográfica física y química de la zona de canales y fiordos australes de Chile entre el estrecho de Magallanes y cabo de Hornos (Cimar 3 Fiordos). Cienc. Tecnol. Mar. 2003, 26, 19–60. [Google Scholar]
- Cokendolpher, J.; Lanfranco, D. Opiliones from the Cape Horn Archipelago: New southern records for harvestment. J. Arachnol. 1985, 13, 311–319. [Google Scholar]
- Henríquez, V.; Licandeo, R.; Cubillos, L.; Cox, S. Interactions between ageing error and selectivity in statistical catch-at-age models: Simulations and implications for assessment of the Chilean Patagonian toothfish fishery. J. Mar. Sci. 2016, 73, 1074–1090. [Google Scholar] [CrossRef] [Green Version]
- Rozzi, R.F.; Massardo, A.; Mansilla, F.; Squeo, E.; Barros, E.; Poulin, M.; Rosenfeld, S.; Goffinet, C.; González-Weaver, R.; Mackenzie, R.D.; et al. Parque Marino Cabo de Hornos–Diego Ramírez. Informe Técnico para la Propuesta de Creación. Punta Arenas, Chile. 2017. Available online: https://issuu.com/umag9/docs/libro_informe_cabo_de_hornos_diego_ (accessed on 2 July 2018).
- Reyes, P. Presencia de corales de aguas frías (Cnidaria: Anthozoa & Hydrozoa) en aguas profundas (306–2.250 m) de la región de Magallanes, Chile. An. Inst. Patagon. 2019, 47, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Stevens, B.; Jewett, S. Growth, molting, and feeding of king crabs. In King Crabs of the World: Biology and Fisheries Management; Stevens, B., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 315–364. [Google Scholar] [CrossRef]
- Lovrich, G.; Vinuesa, J. Biología de las centollas (Anomura: Lithodidae) In Los Crustáceos de Interés Pesquero y Otras Especies Relevantes en los Ecosistemas Marinos; Boschi, E.E., Ed.; INIDEP: Mar del Plata, Argentina, 2016; Volume 6, pp. 183–212. ISBN 978-987-1443-11-6. [Google Scholar]
- Yañez, A. Estatus y Posibilidades de Explotación Biológicamente Sustentables de los Principales Recursos Pesqueros Nacionales, Jaiba y Centolla. Documento Consolidado. Convenio de Desempeño, 2016; IFOP: Valparaíso, Chile, 2017; Available online: https://www.ifop.cl/wp-content/contenidos/uploads/RepositorioIfop/InformeFinal/P-483253_jaiba_centolla.pdf (accessed on 6 May 2020).
- Balzi, P. Ecología y Biología de la Reproducción de la Centolla Lithodes santolla del Golfo de San Jorge. Ph.D. Thesis, Universidad Nacional de la Patagonia San Juan Bosco, Comodoro Rivadavia, CHT, 2005. Available online: https://aquadocs.org/handle/1834/14435 (accessed on 10 February 2020).
- Brey, T. Virtual Handbook. Available online: http://www.thomas-brey.de/science/virtualhandbook/spreadsheets/index.html (accessed on 12 December 2018).
- Hynes, H. The Food of Fresh-Water Sticklebacks (Gasterosteus aculeatus and Pygosteus pungitius), with a Review of Methods Used in Studies of the Food of Fishes. J. Anim. Ecol. 1950, 19, 36–58. [Google Scholar] [CrossRef]
- Brun, E. Food and Feeding Habits of Luidia ciliaris Echinodermata: Asteroidea. J. Mar. Biol. Assoc. U.K. 1972, 52, 225–236. [Google Scholar] [CrossRef]
- Fratt, D.; Dearborn, J. Feeding biology of the Antarctic brittle star Ophionotus victoriae (Echinodermata: Ophiuroidea). Polar Biol. 1984, 3, 127–139. [Google Scholar] [CrossRef]
- Dearborn, J.; Ferrari, F.; Edwards, K. Can pelagic aggregations cause benthic satiation? Feeding biology of the antarctic brittle star Astrotoma agassizii (Echinodermata: Ophiuroidea). In Biology of the Antarctic Seas XVII; Kornicker, L., Ed.; American Geophysical Union: Washington, DC, USA, 1986; Volume 44, pp. 1–28. ISBN 978-1-118-66670-8. [Google Scholar]
- Häussermann, V.; Försterra, G. Marine Benthic Fauna of Chilean Patagonia: Illustrated Identification Guide; Nature in Focus: Puerto Montt, Chile, 2009; p. 1000. ISBN 9789563322446. [Google Scholar]
- Galea, H.R. Hydroids and hydromedusae (Cnidaria: Hydrozoa) from the fjords region of southern Chile. Zootaxa 2007, 1597, 1–116. [Google Scholar]
- Forcelli, D.O. Moluscos Magallánicos: Guía de Moluscos de Patagonia y sur de Chile; Vásquez Mazzini Editores: Buenos Aires, Argentina, 2000; p. 200. ISBN 9879132017. [Google Scholar]
- Fry, B. Food web structure on Georges Bank from stable C, N, and S isotopic compositions. Limnol. Oceanogr. 1988, 33, 1182–1190. [Google Scholar] [CrossRef]
- Jacob, U.; Mintenbeck, K.; Brey, T.; Knust, R.; Beyer, K. Stable isotope food web studies: A case for standardized sample treatment. Mar. Ecol. Prog. Ser. 2005, 287, 251–253. [Google Scholar] [CrossRef] [Green Version]
- Bodin, N.; Le Loc’h, F.; Hily, C. Effect of lipid removal on carbon and nitrogen stable isotope ratios in crustacean tissues. J. Exp. Mar. Biol. Ecol. 2007, 341, 168–175. [Google Scholar] [CrossRef]
- Kiljunen, M.; Grey, J.; Sinisalo, T.; Harrod, C.; Immonen, H.; Jones, R. A revised model for lipid-normalizing δ¹³C values from aquatic organisms, with implications for isotope mixing models. J. Appl. Ecol. 2006, 46, 1213–1222. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial Plymouth.: PRIMER-E 2015. Available online: http://updates.primer-e.com/primer7/manuals/Getting_started_with_PRIMER_7.pdf (accessed on 20 November 2021).
- Hammer, O.; Harper, D.; Ryan, P. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria. 2020. Available online: https://www.R-project.org/ (accessed on 10 March 2020).
- Parnell, A.; Inger, R.; Bearhop, S.; Jackson, A. Source partitioning using stable isotopes: Coping with too much variation. PLoS ONE 2010, 5, e9672. [Google Scholar] [CrossRef]
- McCutchan, J.H.; Lewis, W.M.; Kendall, C.; McGrath, C.C. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 2003, 102, 378–390. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef]
- Comoglio, L.I.; Vinuesa, J.H.; Lovrich, G.A. Feeding habits of southern king crab, Lithodes santolla (Molina), and the false king crab, Paralomis granulosa Jacquinot, in the Beagle Channel. In Proceedings of the International King & Tanner Crabs Symposium, Anchorage, AK, USA, 28–30 November 1989; pp. 315–325. [Google Scholar]
- Adami, M.L.; Gordillo, S. Structure and dynamics of the biota associated with Macrocystis pyrifera (Phaeophyta) from the Beagle Channel, Tierra del Fuego. Sci. Mar. 1999, 63, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Lesser, J.S.; James, W.R.; Stallings, C.D.; Wilson, R.M.; Nelson, J.A. Trophic niche size and overlap decreases with increasing ecosystem productivity. Oikos 2020, 129, 1303–1313. [Google Scholar] [CrossRef]
- Vinuesa, J.H. Efectos e incidencia del parasitismo en la centolla (Lithodes santolla) y el centollón (Paralomis granulosa) del Canal de Beagle. Physis Seccion A 1989, 47, 45–51. [Google Scholar]
- Hines, A.H.; Lipcius, R.N.; Haddon, A.M. Population dynamics and habitat partitioning by size, sex, and molt stage of blue crabs Callinectes sapidus in a subestuary of central Chesapeake Bay. Mar. Ecol. Prog. Ser. 1987, 36, 55–64. [Google Scholar] [CrossRef]
- Araújo, M.S.L.C.; Barreto, A.V.; Negromonte, A.O.; Schwamborn, R. Population ecology of the blue crab Callinectes danae (Crustacea: Portunidae) in a Brazilian tropical estuary. An. Acad. Bras. Cienc. 2012, 84, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, J.H.; Balzi, P. Reproductive biology of Lithodes santolla in the San Jorge Gulf, Argentina. In Crabs in Cold Water Regions: Biology, Management, and Economics; Paul, A.J., Dawe, E.G., Elner, R., Jamieson, G.S., Kruse, G.H., Otto, R.S., Sainte-Marie, B., Shirley, T.C., Woodby, D., Eds.; University of Alaska Sea Grant: Fairbanks, AK, USA, 2002; pp. 283–304. ISBN 978-1-56612-077-7. [Google Scholar]
- Comoglio, L.; Amin, O. Feeding habits of the false southern king crab Paralomis granulosa (Lithodidae) in the Beagle Channel, Tierra del Fuego, Argentina. Sci. Mar. 1999, 63, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.N.; Herrkind, W.F. Predation on early juvenile spiny lobsters Panulirus argus (Latreille): Influence of size and shelter. J. Exp. Mar. Biol. Ecol. 1992, 157, 3–18. [Google Scholar] [CrossRef]
- Halpern, B.S. Habitat bottlenecks in stage-structured species: Hermit crabs as a model system. Mar. Ecol. Prog. Ser. 2004, 276, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Diehl, S. The evolution and maintenance of omnivory: Dynamic constraints and the role of food quality. Ecology 2003, 84, 2557–2567. [Google Scholar] [CrossRef]
- Olsson, K.; Nyström, P.; Stenroth, P.; Nilsson, E.; Svensson, M.; Granéli, W. The influence of food quality and availability on trophic position, carbon signature, and growth rate of an omnivorous crayfish. Can. J. Fish. Aquat. Sci. 2008, 65, 2293–2304. [Google Scholar] [CrossRef]
- Andrade, C.; Ovando, F. First record of microplastics in stomach content of the southern king crab Lithodes santolla (Anomura: Lithodidae), Nassau bay, Cape Horn, Chile. An. Inst. Patagon. 2017, 45, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.; Park, B.; Palace, V. Microplastics in aquatic environments: Implications for Canadian ecosystems. Environ. Pollut. 2016, 218, 269–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, F. and Cowie, P. Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Mar. Pollut. Bull. 2011, 62, 1207–1217. [Google Scholar] [CrossRef]
- Ibañez, C.; Chong, J. Feeding ecology of Enteroctopus megalocyathus (Cephalopoda: Octopodidae) in southern Chile. J. Mar. Biol. Assoc. U.K. 2008, 88, 793–798. [Google Scholar] [CrossRef]
- Golikov, A.V.; Ceia, F.R.; Sabirov, R.M.; Batalin, G.A.; Blicher, M.E.; Gareev, B.I.; Gudmundsson, G.; Jørgensen, L.L.; Mingazov, G.Z.; Zakharov, D.V.; et al. Diet and life history reduce interspecific and intraspecific competition among three sympatric Arctic cephalopods. Sci. Rep. 2020, 10, 21506. [Google Scholar] [CrossRef]
- Cari, I.; Andrade, C.; Quiroga, E.; Mutschke, E. Benthic trophic structure of a Patagonian fjord (47°S): The role of hydrographic conditions in the food supply in a glaciofluvial system. Estuar. Coast. Shelf Sci. 2020, 233. [Google Scholar] [CrossRef]
- Kremmer, B.P. C4-Metabolism in marine brown macrophytic algae. Z. Naturforsch. 1981, 36, 840–847. [Google Scholar] [CrossRef]
- Castilla, J.C.; Moreno, C.A. Sea urchins and Macrocystis pyrifera: Experimental test of their ecological relations in southern Chile. In Proceedings of the International Conference, Tampa Bay, FL, USA, 14–17 September 1981; pp. 257–263. [Google Scholar]
- Castilla, J.C. Food webs and functional aspects of the kelp, Macrocystis pyrifera, community in the Beagle Channel, Chile. In Antarctic Nutrient Cycles and Food Webs; Siegfried, W.R., Condy, P.R., Laws, R.M., Eds.; Springer: Berlin, Germany, 1985; pp. 407–414. ISBN 978-3-642-82275-9. [Google Scholar]
- Ojeda, F.P.; Santelices, B. Invertebrate communities in holdfasts of the kelp Macrocystis pyrifera from southern Chile. Mar. Ecol. Prog. Ser. 1984, 16, 65–73. [Google Scholar] [CrossRef]
- Pessarrodona, A.; Moore, P.J.; Sayer, M.D.; Smale, D.A. Carbon assimilation and transfer through kelp forests in the NE Atlantic is diminished under a warmer ocean climate. Glob. Chang. Biol. 2018, 24, 4386–4398. [Google Scholar] [CrossRef] [Green Version]
- Dunton, K.H.; Schell, D.M. Dependence of consumers on macroalgal (Laminaria solidungula) carbon in an arctic kelp community: δ¹³C evidence. Mar. Biol. 1987, 93, 615–625. [Google Scholar] [CrossRef]
- Mann, K.H. Production and use of detritus in various freshwater, estuarine, and coastal marine ecosystems. Limnol. Oceanogr. 1988, 33, 910–930. [Google Scholar]
- Harrold, C.; Light, K.; Lisin, S. Organic enrichment of submarine-canyon and continental-shelf benthic communities by macroalgal drift imported from nearshore kelp forests. Limnol. Oceanogr. 1998, 43, 669–678. [Google Scholar] [CrossRef]
- Polis, G.A.; Strong, D.R. Food web complexity and community dynamics. Am. Nat. 1996, 147, 813–846. [Google Scholar] [CrossRef]
- Sterner, R.W. Modelling interactions of food quality and quantity in homeostatic consumers. Freshw. Biol. 1997, 38, 473–481. [Google Scholar] [CrossRef]
- Mitra, A.; Flyn, K.J. Importance of interactions between food quality, quantity, and gut transit time on consumer feeding, growth, and trophic dynamics. Am. Nat. 2007, 196, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Richoux, N.B.; Bergamino, L.; Moyo, S.; Dalu, T. Spatial and temporal variability in the nutritional quality of basal resources along a temperate river/estuary continuum. Org. Geochem. 2018, 116, 1–12. [Google Scholar] [CrossRef]
- Andrade, C.; Ríos, C.; Gerdes, D.; Brey, T. Trophic structure of shallow-water benthic communities in the sub-Antarctic Strait of Magellan. Polar Biol. 2016, 39, 2281–2297. [Google Scholar] [CrossRef] [Green Version]
- Riccialdelli, L.; Newsome, S.D.; Fogel, M.L.; Fernández, D.A. Trophic interactions and food web structure of a subantarctic marine food web in the Beagle Channel: Bahía Lapataia, Argentina. Polar Biol. 2016, 40, 807–821. [Google Scholar] [CrossRef]
- Kaehler, S.; Pakhomov, E.A.; Kalin, R.M.; Davis, S. Trophic importance of kelp-derived suspended particulate matter in a through-flow sub-Antarctic system. Mar. Ecol. Prog. Ser. 2006, 316, 17–22. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, M.A.Z.; de Freitas, S.C.; Berneira, L.M.; Mansilla, A.; Astorga-España, M.S.; Colepicolo, P.; Pereira, C.M. Pigment concentration, photosynthetic performance, and fatty acid profile of sub-Antarctic brown macroalgae in different phases of development from the Magellan Region, Chile. J. Appl. Phycol. 2019, 31, 2629–2642. [Google Scholar] [CrossRef]
- Guo, F.; Kainz, M.J.; Sheldon, F.; Bunn, S.E. The importance of high-quality algal food sources in stream food webs—Current status and future perspectives. Freshw. Biol. 2016, 61, 815–831. [Google Scholar] [CrossRef]
- Werner, E.E.; Gilliam, J.F. The ontogenetic niche and species interactions in size-structured populations. Ann. Rev. Ecol. Syst. 1984, 15, 393–425. [Google Scholar] [CrossRef]
- Woodward, G.; Hildrew, A.G. Body-size determinants of niche overlap and intraguild predation within a complex food web. J. Anim. Ecol. 2002, 71, 1063–1074. [Google Scholar] [CrossRef]
- Polis, G.A. Age Structure Component of Niche Width and Intraspecific Resource Partitioning: Can Age Groups Function as Ecological Species? Am. Nat. 1984, 123, 541–564. [Google Scholar] [CrossRef]
- Fokkema, W.; van der Jeugd, H.P.; Lameris, T.K.; Dokter, A.M.; Ebbinge, B.S.; Ross, A.M.; Nolet, A.N.; Piersma, T. Ontogenetic niche shifts as a driver of seasonal migration. Oecologia 2020, 193, 285–297. [Google Scholar] [CrossRef]
- Bearhop, S.; Phillips, R.A.; McGill, R.; Cherel, Y.; Dawson, D.A.; Croxall, J.P. Stable isotopes indicate sex-specific and long-term individual foraging specialisation in diving seabirds. Mar. Ecol. Prog. Ser. 2006, 311, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Lovrich, G.A. La pesquería mixta de las centollas Lithodes santolla y Paralomis granulosa (Anomura: Lithodidae) en Tierra del Fuego, Argentina. Investig. Mar. 1997, 25, 41–57. [Google Scholar] [CrossRef] [Green Version]
- Lovrich, G.L.; Vinuesa, J.H.; Smith, B.D. Growth, maturity, and mating of male southern king crab (Lithodes santolla) in the Beagle Channel, Argentina. In Crabs in Cold Water Regions: Biology, Management, and Economics; Paul, A.J., Dawe, E.G., Elner, R., Jamieson, G.S., Kruse, G.H., Otto, R.S., Sainte-Marie, B., Shirley, T.C., Woodby, D., Eds.; University of Alaska Sea Grant: Fairbanks, AK, USA, 2002; pp. 147–168. ISBN 978-1-56612-077-7. [Google Scholar]
- Pianka, E.R. Competition and niche theory, In Theoretical Ecology: Principles and Applications, 2nd ed.; May, R.M., Ed.; Blackwell Scientific: Oxford, UK, 1981; pp. 167–196. [Google Scholar]
- Bolnick, D.I.; Svanbäck, R.; Fordyce, J.A.; Yang, L.H.; Davis, J.M.; Hulsey, C.D.; Forister, M.L.; McPeek, M.A. The ecology of individuals: Incidence and implications of individual specialization. Am. Nat. 2003, 161, 1–28. [Google Scholar] [CrossRef]
- Chaguaceda, F.; Eklöv, P.; Scharnweber, K. Regulation of fatty acid composition related to ontogenetic changes and niche differentiation of a common aquatic consumer. Oecologia 2020, 193, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Ríos, C. Marine Benthic Communities of the Magellan Region, Southern Chile: Contributions of Different Habitats to the Overall Biodiversity. Ph.D. Thesis, University of Bremen, Bremen, Germany, 2007. Available online: https://media.suub.uni-bremen.de/handle/elib/2516 (accessed on 10 February 2020).
- Lovrich, G.; Sainte-Marie, B. Cannibalism in the snow crab, Chionoecetes opilio (O. Fabricius) (Brachyura: Majidae), and its potential importance to recruitment. J. Exp. Mar. Biol. Ecol. 1997, 211, 225–245. [Google Scholar] [CrossRef]
- Marshall, S.; Warburton, K.; Peterson, B.; Mann, D. Cannibalism in juvenile blue-swimmer crabs Portunus pelagicus (Linnaeus, 1766): Effects of body size, moult stage and refuge availability. Appl. Anim. Behav. Sci. 2005, 90, 65–82. [Google Scholar] [CrossRef]
- Fry, B.; Arnold, C. Rapid ¹³C/¹²C turnover during growth of brown shrimp (Penaeus aztecus). Oecologia 1982, 54, 200–204. [Google Scholar] [CrossRef]
- Fry, B. Stable Isotope Ecology; Springer: New York, NY, USA, 2006; p. 308. ISBN 978-0-387-33745-6. [Google Scholar]
- Vander Zanden, M.J.; Clayton, M.K.; Moody, E.K.; Solomon, C.T.; Weidel, B.C. Stable isotope turnover and half-life in animal tissues: A literature synthesis. PLoS ONE 2015, 10, e0116182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hentschel, B.T. Intraspecific variations in δ¹³C indicate ontogenetic diet changes in deposit-feeding polychaetes. Ecology 1998, 79, 1357–1370. [Google Scholar] [CrossRef]
- Lim, S.S.; Yong, A.Y.; Christy, J.H. Ontogenetic changes in diet and related morphological adaptations in Ocypode gaudichaudii. Invertebr. Biol. 2016, 135, 117–126. [Google Scholar] [CrossRef]
- Mancinelli, G.; Glamuzina, B.; Petric, M.; Carrozzo, L.; Glamuzina, L.; Zotti, M.; Raho, D.; Vizzini, S. The trophic position of the Atlantic blue crab Callinectes sapidus Rathbun 1896 in the food web of Parila Lagoon (South Eastern Adriatic, Croatia): A first assessment using stable isotopes. Mediterr. Mar. Sci. 2016, 17, 634–643. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Jeppesen, E.; Gu, X.; Mao, Z.; Chen, H. Cannibalism and habitat selection of cultured Chinese Mitten Crab: Effects of submerged aquatic vegetation with different nutritional and refuge values. Water 2018, 10, 1542. [Google Scholar] [CrossRef] [Green Version]
- Bigatti, G.; Sanchez, C.; Miloslavich, P.; Penchaszadeh, P.E. Feeding behavior of Adelomelon ancilla (Ligfoot, 1786): A predatory neogastropod (Gastropoda: Volutidae) in Patagonian benthic communities. Nautilus 2009, 123, 159–165. [Google Scholar]
- Vögler, R.; Milessi, A.; Quiñones, R. Influence of environmental variables on the distribution of Squatina guggenheim (Chondrichthyes, Squatinidae) in the Argentine–Uruguayan Common Fishing Zone. Fish. Res. 2008, 91, 212–221. [Google Scholar] [CrossRef]
- Benke, A.C.; Wallace, J.B.; Harrison, J.W.; Koebel, J.W. Food web quantification using secondary production analysis: Predaceous invertebrates of the snag habitat in a subtropical river. Freshw. Biol. 2001, 46, 329–346. [Google Scholar] [CrossRef]
- Coll, M.; Guershon, M. Omnivory in terrestrial arthropods: Mixing plant and prey diets. Annu. Rev. Entomol. 2002, 47, 267–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| N | Length (CL mm) | Width (mm) | Weight (gr) | Sampling Method | |
|---|---|---|---|---|---|
| Lithodes santolla Adult Males | 46 | 88–163 | 90–175 | 600–3200 | Crab traps |
| Lithodes santolla Adult Females | 45 | 90–140 | 94–145 | 500–2000 | Crab traps |
| Lithodes santolla Juvenile Males | 30 | 54–79 | 54–84 | 95–354 | Crab traps |
| Lithodes santolla Juvenile Females | 28 | 56–80 | 58–85 | 100–355 | Crab traps |
| Lithodes santolla All | 149 | 54–163 | 54–175 | 95–3200 | Crab traps |
| Prey Groups | Adult | SD | Adult | SD | Juvenile | SD | Juvenile | SD | All | SD |
|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | |||||||
| Algae | 23.8 | 37.01 | 9 | 13.4 | 7.7 | 14.4 | 6.3 | 12.2 | 12.3 | 23.4 |
| Hydrozoa | 11.3 | 27.3 | 20.1 | 25.6 | 6.7 | 12.6 | 10.8 | 15.8 | 13.2 | 22.8 |
| Bryozoa | 5 | 17.2 | 14 | 27.5 | 1.2 | 4 | 3.1 | 9.1 | 6.8 | 19.2 |
| Porifera | - | - | - | - | 3.4 | 14.8 | 14.9 | 32 | 3.6 | 16.5 |
| Foraminifera | 0.5 | 1.8 | 2.5 | 2.6 | 4.7 | 6 | 9.1 | 2.7 | 5.3 | |
| Polychaeta | 1.9 | 5.1 | 2.5 | 5.8 | 4.8 | 14.1 | 0.7 | 3.7 | 2.4 | 7.8 |
| Echinodermata | 3.1 | 16.7 | 3.8 | 10.2 | 10.6 | 23.2 | 1.4 | 4.1 | 4.5 | 15.1 |
| Crustacea | 31.9 | 40.2 | 12.6 | 22.4 | 19.7 | 23.2 | 10.6 | 18 | 18.9 | 28.9 |
| Bivalvia | 5.9 | 20.1 | 12.6 | 20.9 | 17.1 | 17.6 | 24.3 | 33 | 14 | 23.7 |
| Gastropoda | 0.5 | 2.3 | 0.4 | 1.6 | 0.7 | 3.7 | 1.2 | 4.3 | 0.7 | 2.9 |
| Cephalopoda | 3 | 11 | - | - | - | - | - | - | 0.8 | 5.9 |
| Fish | 10.9 | 23.6 | 15.3 | 27.0 | 6.5 | 20.8 | 9.1 | 19.9 | 11.1 | 24 |
| Plastic | 2.1 | 3 | 6.5 | 16.8 | 11.5 | 23 | 9.6 | 15.5 | 6.9 | 16 |
| Sediment | - | - | 0 | 0 | 3.6 | 12.6 | 0 | 0 | 0.7 | 5.7 |
| Detritus | - | - | 0.1 | 1 | 3.8 | 19.6 | 1.9 | 6.1 | 1.2 | 9.1 |
| Other | - | - | 0.6 | 3 | - | - | - | - | 0.2 | 1.7 |
| Number of | ||||||||||
| stomachs with food | 36 | 43 | 26 | 26 | 131 |
| Source of Variation | df | Sums of Square | Pseudo-F | F (Perm) | Perm |
|---|---|---|---|---|---|
| Maturity (M) | 1 | 12458 | 3.6216 | 0.001 | 999 |
| Sex (S) | 1 | 7526.5 | 2.1879 | 0.014 | 999 |
| M × S | 1 | 5184.5 | 1.5071 | 0.111 | 999 |
| Res | 127 | 3440.1 | |||
| Total | 130 |
| ITEMS | Av.Abund | AvAbund | AvDiss | Diss/SD | Contrib% | Cum% |
|---|---|---|---|---|---|---|
| Adults | Juveniles | |||||
| Crustaceans | 6.17 | 1.57 | 12 | 1.81 | 19.84 | 19.84 |
| Hydrozoans | 1.76 | 4.07 | 8.49 | 1.31 | 14.05 | 33.89 |
| Bivalvia | 2.69 | 4.64 | 6.85 | 1.29 | 11.34 | 45.23 |
| Algae | 3.61 | 2.55 | 6.65 | 1.18 | 10.99 | 56.22 |
| Average Dissimilarity 83.0 | ||||||
| ITEMS | AvAbund | AvAbund | AvDiss | Diss/SD | Contrib% | Cum% |
|---|---|---|---|---|---|---|
| Male | Female | |||||
| Crustaceans | 18.37 | 12.64 | 11.92 | 0.83 | 14.5 | 14.5 |
| Hydrozoans | 10.31 | 16.84 | 11.14 | 0.77 | 13.55 | 28.04 |
| Algae | 14.09 | 10.45 | 10.15 | 0.75 | 12.35 | 40.39 |
| Bivalvia | 11.39 | 13.05 | 9.03 | 0.75 | 10.99 | 51.38 |
| Fish | 8.69 | 9.43 | 8.22 | 0.59 | 10 | 61.38 |
| Average Dissimilarity 81.2 | ||||||
| Phyla/Taxon | N | δ15N Range | Mean δ15N | SD | δ13C Range | Mean δ13C | SD | TP | Diet | Feeding Mode | Sample Type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arthropoda | |||||||||||
| Crustacea | |||||||||||
| Lithodes santolla Adults | 60 | 10.5–12.6 | 11.6 | 0.4 | −16.9–−13.3 | −14.7 | 0.8 | 3.3 | Om | Pr | MT |
| Lithodes santolla Juveniles | 28 | 10.9–12.6 | 11.4 | 0.3 | −16.4–−14.3 | −15.3 | 0.5 | 3.3 | Om | Pr | MT |
| Lithodes santolla Males | 44 | 11.2–12.6 | 11.7 | 0.4 | −16.9–−13.3 | −15.1 | 0.8 | 3.4 | Om | Pr | MT |
| Lithodes santolla Females | 44 | 10.5–12.2 | 11.4 | 0.3 | −16.4–−13.7 | −14.8 | 0.7 | 3.2 | Om | Pr | MT |
| Lithodes santolla All | 88 | 10.5–12.6 | 11.6 | 0.4 | −16.9–−13.3 | −14.9 | 0.8 | 3.3 | Om | Pr | MT |
| Paralomis granulosa | 3 | 10.4–11.5 | 11 | 0.6 | −14.7–−14.6 | −14.7 | 0.1 | 3.1 | Om | Pr | MT |
| Propagurus gaudichaudi | 3 | 12.3–12.7 | 12.5 | 0.2 | −16.3–−15.1 | −15.8 | 0.6 | 3.7 | Om | Sc, DF | MT |
| Eurypodius sp. | 3 | 11.7–12.8 | 12.3 | 0.5 | −15.8–−14.6 | −15.2 | 0.6 | 3.6 | Om | Sc, DF | MT |
| Austromegabalanus psittacus | 1 | 8.7 | −19.1 | 2.1 | Om | SF | ST, a | ||||
| Mollusca | |||||||||||
| Cephalopoda | |||||||||||
| Enteroctopus megalocyathus | 6 | 13.4–14.2 | 13.7 | 0.4 | −16.6–−15.7 | −16.1 | 0.4 | 4.2 | Ca | Pr | MT |
| Gastropoda | |||||||||||
| Adelomelon ancilla | 4 | 12.7–13.3 | 13 | 0.3 | −15.5–−15.2 | −15.4 | 0.1 | 3.9 | Ca | Pr | MT, a |
| Echinodermata | |||||||||||
| Asteroidea | |||||||||||
| Cosmasterias lurida | 6 | 11.4–12.4 | 12 | 0.5 | −16.8–−14.1 | −15.9 | 1 | 3.5 | Ca | Pr | TF, a |
| Chordata | |||||||||||
| Ascidiacea | |||||||||||
| Ascidia indet. | 1 | 9.1 | −20.3 | 2.2 | Om | SF | ST, a | ||||
| Actinopterygii | |||||||||||
| Zoarcidae | |||||||||||
| Crossostomus chilensis | 4 | 10.4–12.3 | 11.7 | 0.9 | −15.4–−14.7 | −15 | 0.3 | 3.4 | Ca | Pr | MT, b |
| Porifera | |||||||||||
| Mycale sp. | 1 | 8.9 | –19 | 2.1 | Om | SF | ST, a | ||||
| Haliclona sp. | 2 | 8.2–9.1 | 8.6 | 0.6 | −19.8–−18 | −18.9 | 1.3 | 2 | Om | SF | ST, a |
| Primary producers | |||||||||||
| Rhodophyta | |||||||||||
| Gigartina skottsbergii | 3 | 6.5–6.7 | 6.6 | 0.1 | −23.2–−22.4 | −22.8 | 0.4 | Aut | a | ||
| Gracilaria chilensis | 5 | 4.3–6.6 | 5.8 | 1 | −30.5–−28.9 | −29.5 | 0.6 | Aut | a | ||
| Porphyra columbina | 2 | 6.7–8.1 | 7.4 | 0.9 | −21.9–−19.9 | −20.9 | 1.4 | Aut | a | ||
| Chlorophyta | |||||||||||
| Ulva lactuca | 3 | 6.4–7 | 6.7 | 0.3 | −23.7–−23.1 | −23.4 | 0.3 | Aut | a | ||
| Ochrophyta | |||||||||||
| Macrocystis pyrifera | 3 | 6.1–6.5 | 6.3 | 0.2 | −15.6–−15.6 | −15.6 | 0.00 | Aut | a | ||
| Sediment | 4 | 4.9–6.4 | 5.6 | 0.7 | −18.6–−18 | −18.3 | 0.3 | a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, C.; Rivera, C.; Daza, E.; Almonacid, E.; Ovando, F.; Morello, F.; Pardo, L.M. Trophic Niche Dynamics and Diet Partitioning of King Crab Lithodes santolla in Chile’s Sub-Antarctic Water. Diversity 2022, 14, 56. https://doi.org/10.3390/d14010056
Andrade C, Rivera C, Daza E, Almonacid E, Ovando F, Morello F, Pardo LM. Trophic Niche Dynamics and Diet Partitioning of King Crab Lithodes santolla in Chile’s Sub-Antarctic Water. Diversity. 2022; 14(1):56. https://doi.org/10.3390/d14010056
Chicago/Turabian StyleAndrade, Claudia, Cristóbal Rivera, Erik Daza, Eduardo Almonacid, Fernanda Ovando, Flavia Morello, and Luis Miguel Pardo. 2022. "Trophic Niche Dynamics and Diet Partitioning of King Crab Lithodes santolla in Chile’s Sub-Antarctic Water" Diversity 14, no. 1: 56. https://doi.org/10.3390/d14010056
APA StyleAndrade, C., Rivera, C., Daza, E., Almonacid, E., Ovando, F., Morello, F., & Pardo, L. M. (2022). Trophic Niche Dynamics and Diet Partitioning of King Crab Lithodes santolla in Chile’s Sub-Antarctic Water. Diversity, 14(1), 56. https://doi.org/10.3390/d14010056