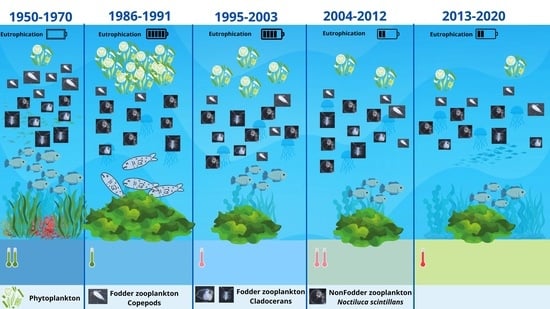
Dynamics of Zooplankton along the Romanian Black Sea Coastline: Temporal Variation, Community Structure, and Environmental Drivers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.2. Zooplankton Sampling and Analysis
2.3. Data Analysis
3. Results
3.1. The Environmental Parameters
3.2. Zooplankton Composition
3.3. Zooplankton Structure–Seasonal Variations
3.4. Zooplankton Structure in Relation to Physicochemical Parameters
4. Discussion
4.1. Zooplankton Composition
4.2. Zooplankton Structure–Seasonal Variations
4.3. Zooplankton Structure in Relation to Physicochemical Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Caroppo, C.; Buttino, I.; Camatti, E.; Caruso, G.; De Angelis, R.; Facca, C.; Magaletti, E. State of the Art and Perspectives on the Use of Planktonic Communities as Indicators of Environmental Status in Relation to the EU Marine Strategy Framework Directive. Biol. Mar. Mediterr. 2013, 20, 65–73. [Google Scholar]
- Kiko, R.; Bianchi, D.; Grenz, C.; Hauss, H.; Iversen, M.; Kumar, S.; Maas, A.; Robinson, C. Editorial: Zooplankton and Nekton: Gatekeepers of the Biological Pump. Front. Mar. Sci. 2020, 7, 545. [Google Scholar] [CrossRef]
- Oguz, T.; Velikova, V. Abrupt Transition of the Northwestern Black Sea Shelf Ecosystem from a Eutrophic to an Alternative Pristine State. Mar. Ecol. Prog. Ser. 2010, 405, 231–242. [Google Scholar] [CrossRef]
- Daskalov, G.M. Overfishing Drives Atrophic Cascade in the Black Sea. Mar. Ecol. Prog. Ser. 2002, 225, 53–63. [Google Scholar] [CrossRef]
- Kideys, A.E.; Kovalev, A.V.; Shulman, G.; Gordina, A.; Bingel, F. A review of zooplankton investigations of the Black Sea over the last decade. J. Mar. Syst. 2000, 24, 355–371. [Google Scholar] [CrossRef]
- Erdogan, N.; Duzgunes, E.; Ogut, H. Black Sea fisheries and climate change. In CIESM Workshop Monographs; CIESM: Monaco, 2009. [Google Scholar]
- Drira, Z.; Kmiha-Megdiche, S.; Sahnoun, H.; Pagano, M.; Tedetti, M.; Ayadi, H. Water Quality Affects the Structure of Copepod Assemblages along the Sfax Southern Coast (Tunisia, Southern Mediterranean Sea). Mar. Freshw. Res. 2018, 69, 220–231. [Google Scholar] [CrossRef]
- Onciu, T.M.; Skolka, M.; Gomoiu, M.T. Ecologia Comunităților Zooplanctonice de la Litoralul Românesc al Mării Negre; Ovidius University Press: Constanța, Romania, 2006. [Google Scholar]
- Liu, H.; Zhang, X.; Yang, Q.; Zuo, T.; Quigg, A. Mesozooplankton Dynamics in Relation to Environmental Factors and Juvenile Fish in a Subtropical Estuary of the Gulf of Mexico. J. Coast. Res. 2017, 335, 1038–1050. [Google Scholar] [CrossRef]
- Richardson, A.J. In Hot Water: Zooplankton and Climate Change. ICES J. Mar. Sci. 2008, 65, 279–295. [Google Scholar] [CrossRef]
- Schroeder, A.; Stanković, D.; Pallavicini, A.; Gionechetti, F.; Pansera, M.; Camatti, E. DNA Metabarcoding and Morphological Analysis—Assessment of Zooplankton Biodiversity in Transitional Waters. Mar. Environ. Res. 2020, 160, 104946. [Google Scholar] [CrossRef]
- Jeelani, M.; Kaur, H.; Kumar, R. Impact of Climate Warming on the Biodiversity of Freshwater Ecosystem of Kashmir, India. In Proceedings of the Taal 2007: The 12th World Lake Conference, Jaipur, Rajasthan, India, 28 October–2 November 2007; Volume 1103, p. 1109. [Google Scholar]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Biodiversity and Ecosystem Functioning: Current Knowledge and Future Challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef]
- Ferrara, O.; Vagaggini, D.; Margaritora, F.G. Zooplankton Abundance and Diversity in Lake Bracciano, Latium, Italy. J. Limnol. 2002, 61, 169–175. [Google Scholar] [CrossRef]
- Jeppesen, E.; Nõges, P.; Davidson, T.A.; Haberman, J.; Nõges, T.; Blank, K.; Lauridsen, T.L.; Søndergaard, M.; Sayer, C.; Laugaste, R.; et al. Zooplankton as Indicators in Lakes: A Scientific-Based Plea for Including Zooplankton in the Ecological Quality Assessment of Lakes According to the European Water Framework Directive (WFD). Hydrobiologia 2011, 676, 279–297. [Google Scholar] [CrossRef]
- Kehayias, G.; Chalkia, E.; Doulka, E. Zooplankton Variation in Five Greek Lakes. In Zooplankton; Nova Science Publisher, Inc.: Hauppauge, NY, USA, 2014. [Google Scholar]
- Preston, N.D.; Rusak, J.A. Homage to Hutchinson: Does Inter-Annual Climate Variability Affect Zooplankton Density and Diversity? Hydrobiologia 2010, 653, 165–177. [Google Scholar] [CrossRef]
- Caroni, R.; Irvine, K. The Potential of Zooplankton Communities for Ecological Assessment of Lakes: Redundant Concept or Political Oversight? Biol. Environ. Proc. R. Ir. Acad. 2010, 110, 35–53. [Google Scholar] [CrossRef]
- Lazăr, L.; Boicenco, L.; Moncheva, S.; Denga, Y.; Atabay, H.; Abaza, V.; Bişinicu, E.; Coatu, V.; Filimon, A.; Harcotă, G.; et al. Impact of the Rivers on the Black Sea Ecosystem; CD Press: Bucharest, Romania, 2021. [Google Scholar]
- Damir, N.A.; Coatu, V.; Pantea, E.D.; Galațchi, M.; Botez, E.; Birghilă, S. Assessment of Polycyclic Aromatic Hydrocarbons Content in Marine Organisms of Commercial Interest from the Romanian Black Sea Coast. Polycycl. Aromat. Compd. 2022, 42, 7595–7606. [Google Scholar] [CrossRef]
- Alexandrov, B.; Arashkevich, E.; Gubanova, A.; Korshenko, A. Manual for Mesozooplankton Sampling and Analysis in the BlackSea Monitoring; Black Sea Commission: Istanbul, Turkey, 2014. [Google Scholar]
- Mordukhay-Boltovskoy, F.D. (Ed.) . Guide of the Black Sea and the Sea of Azov Fauna; Naukova Dumka: Kiev, Ukraine, 1972. [Google Scholar]
- Mordukhay-Boltovskoy, F.D. (Ed.) . Identification Manual on the Fauna of the Black and Azov Seas; Naukova Dumka: Kiev, Ukraine, 1968. [Google Scholar]
- Petipa, T.S. On the Mean Weight of the Principle Forms of Zooplankton in the Black Sea. Sevast. Biol. Stn. 1957, 9, 39–57. [Google Scholar]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis, 3rd ed.; Grasshoff, K., Kremling, K., Ehrhardt, M., Eds.; Willey-VCH: Weinheim, Germany, 1999. [Google Scholar]
- Clarke, K.R.; Gorley, R.N.; Somerfield, P.J. Change in Marine Communities: An Approach to Statistical Analysis, 3rd ed.; PRIMER-E: Plymouth, UK, 2014. [Google Scholar]
- Addinsoft, Inc. Addinsoft XLSTAT Software; Version 2021.2.1; Addinsoft, Inc.: New York, NY, USA, 2021. [Google Scholar]
- TIBCO Software, Inc. TIBCO Statistica; Version 14.0.1.25; TIBCO Software, Inc.: Palo Alto, CA, USA, 2023. [Google Scholar]
- ESRI. ArcGIS Desktop; Version 10.7; Environmental Systems Research Institute: Redlands, CA, USA, 2019. [Google Scholar]
- Gubanova, A.; Altukhov, D. Establishment of Oithona brevicornis Giesbrecht, 1892 (Copepoda: Cyclopoida) in the Black Sea. Aquat. Invasions 2007, 2, 407–410. [Google Scholar] [CrossRef]
- Niette, V. Huliselan. The Role of Zooplankton Predator, Chaetognaths (Sagitta Spp.) in Baguala Bay Waters, Ambon Island. J. Coast. Dev. 2002, 6, 9–21. [Google Scholar]
- Masunaga, A.; Liu, A.W.; Tan, Y.; Scott, A.; Luscombe, N.M. Streamlined Sampling and Cultivation of the Pelagic Cosmopolitan Larvacean. J. Vis. Exp. 2020, 160, e61279. [Google Scholar] [CrossRef]
- Sato, R.; Tanaka, Y.; Ishimaru, T. House Production by Oikopleura dioica (Tunicata, Appendicularia) Under Laboratory Conditions. J. Plankton Res. 2001, 23, 415–423. [Google Scholar] [CrossRef]
- Tiselius, P.; Petersen, J.; Nielsen, T.; Maar, M.; Møller, E.; Satapoomin, S.; Tönnesson, K.; Zervoudaki, T.; Christou, E.; Giannakourou, A.; et al. Functional Response of Oikopleura dioica to House Clogging Due to Exposure to Algae of Different Sizes. Mar. Biol. 2003, 142, 253–261. [Google Scholar] [CrossRef]
- Byrne, P. Seasonal Composition of Meroplankton in the Dunkellin Estuary, Galway Bay. Biol. Environ. Proc. R. Ir. Acad. 1995, 95, 35–48. [Google Scholar]
- Üstün, F.; Bat, L.; Mutlu, E. Seasonal Variation and Taxonomic Composition of Mesozooplankton in the Southern Black Sea (off Sinop) between 2005 and 2009. Turk. J. Zool. 2018, 42, 541–556. [Google Scholar] [CrossRef]
- Kıdeyş, A.E.; Bingel, F.; Niermann, U. The Effect of Environmental conditions on the Distribution of Eggs and Larvae of Anchovy (Engraulis encrasicolus L.) in the Black Sea. ICES J. Mar. Sci. 1999, 24, 58–64. [Google Scholar] [CrossRef]
- Shiganova, T.; Musaeva, E.; Arashkevich, E.; Shirshov, P.P.; Kamburska, L.; Stefanova, K.; Mihneva, V.; Polishchuk, L.; Timofte, F.; Ustun, F.; et al. The State of Zooplankton. In State of the Environment of the Black Sea (2001–2006/7); Oguz, T., Ed.; The Commission on the Protection of the Black Sea Against Pollution Publication: Istanbul, Turkey, 2007; pp. 201–246. [Google Scholar]
- Porumb, F. Le Zooplancton de La Mer Noire. Biologie Des Eaux Saumâtres de La Mer Noire. IRCM 1977, 1, 99–108. [Google Scholar]
- Porumb, F. L’Histoire Des Recherches Marines Roumaines En Mer Noire; Inst. Roman de Cercetari Marine: Constanta, Romania, 1995; pp. 32–33. [Google Scholar]
- Zaitsev, Y. Recent Changes in the Trophic Structure of the Black Sea. Fish. Oceanogr. 1992, 1, 180–189. [Google Scholar] [CrossRef]
- Kamburska, L.; Schrimpf, W.; Djavidnia, S.; Shiganova, T.; Stefanova, K. Adressing the Ecological Issue of the Invasive Species Special Focus on the Ctenophore Mnemiopsis Leidy (Agassiz, 1865) in the Black Sea; Office for Official Publications of the European Communities: Luxembourg, 2006. [Google Scholar]
- Kovalev, A.V.; Finenko, Z.Z. Macrozooplankton: Plankton of the Black Sea; Naukova Dumka: Kiev, Ukraine, 1993; pp. 183–193. [Google Scholar]
- Polischuk, L.N.; Nastenko, E.V.; Garkavaya, G.P. Some Peculiarities of Modern State of Pelagic and Neustonic Zoocenosis of the Black Sea. Ecol. Morya 1984, 18, 25–34. [Google Scholar]
- Shiganova, T.A.; Shirshov, P.P. Invasion of the Black Sea by the Ctenophore Mnemiopsis leidyi and Recent Changes in Pelagic Community Structure. Fish. Oceanogr. 1998, 7, 305–310. [Google Scholar] [CrossRef]
- Konsutov, A.S.; Kamburska, L.T. Ecological Determination of the New Ctenophora-Beroe ovata invasion in the Black Sea. Oceanology 1998, 2, 195–198. [Google Scholar]
- Shiganova, T.A.; Dumont, H.J.; Mikaelyan, A.; Glazov, D.M.; Bulgakova, Y.V.; Musaeva, E.I.; Studenikina, E. Interaction between the Invading Ctenophores Mnemiopsis Leidyi (A. Agassiz) and Beroe ovata Mayer 1912, and Their Influence on the Pelagic Ecosystem of the Northeastern Black Sea. In Aquatic Invasions in the Black, Caspian, and Mediterranean Seas; Dumont, H., Shiganova, T.A., Niermann, U., Eds.; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 2004; Volume 35, pp. 33–70. [Google Scholar]
- Oguz, T.; Dippner, J.W.; Kaymaz, Z. Climatic Regulation of the Black Sea Hydro-Meteorological and Ecological Properties at Interannual-to-Decadal Time Scales. J. Mar. Syst. 2006, 60, 235–254. [Google Scholar] [CrossRef]
- Vargas, C.A.; Martínez, R.A.; Escribano, R.; Lagos, N.A. Seasonal Relative Influence of Food Quantity, Quality, and Feeding Behaviour on Zooplankton Growth Regulation in Coastal Food Webs. J. Mar. Biol. Assoc. U. K. 2010, 90, 1189–1201. [Google Scholar] [CrossRef]
- Petran, A.; Moldoveanu, M. Post-Invasion Ecological Impact of The Atlantic Ctenophore Mnemiopsis leidyi Agassiz, 1865 On the Zooplankton from The Romanian Black Sea Waters. Cercet. Mar. 1995, 27–28, 135–157. [Google Scholar]
- Shiganova, T.; Stupnikova, A.; Stefanova, K. Genetic Analyses of Non-Native Species Oithona davisae Ferrari, F.D. & Orsi, 1984 in the Black Sea. Bioinvasions Rec. 2015, 4, 91–95. [Google Scholar] [CrossRef]
- Tkach, A.V. Changes in the Larvae Nutrition of the Black Sea Fishes with Respect to Plankton. NATO Sci. Ser. 2 Environ. Secur. 1997, 47, 235–248. [Google Scholar]
- Timofte, F.; Tabarcea, C. Oithona brevicornis Giesbrecht, 1892 (Copepoda: Cyclopoida)—First Record in the Romanian Black Sea Waters. J. Environ. Prot. Ecol. 2012, 13, 1683–1687. [Google Scholar]
- Islam, M.S.; Azadi, M.A.; Nasiruddin, M.; Sarker, M.M. Plankton Species Composition, Abundance and Diversity Indices In Three Ponds Of Chittagong University Campus, Bangladesh. Fish. Aquac. J. 2022, 14, 321. [Google Scholar]
- Kovalev, A.V.; Mazzocchi, M.G.; Siokou-Frangou, I.; Kideys, A.E. Zooplankton of the Black Sea and the Eastern Mediterranean: Similarities and Dissimilarities. Mediterr. Mar. Sci. 2001, 2, 69–77. [Google Scholar] [CrossRef]
- Leppäkoski, E.; Gollasch, S.; Gruszka, P.; Ojaveer, H.; Olenin, S.; Panov, V. The Baltic—A Sea of Invaders. Can. J. Fish. Aquat. Sci. 2002, 7, 1175–1188. [Google Scholar] [CrossRef]
- Oğuz, T.; Öztürk, B. Mechanisms Impeding Natural Mediterranization Process of Black Sea Fauna. J. Black Sea Mediterr. Environ. 2011, 17, 234–253. [Google Scholar]
- Moldoveanu, M.; Timofte, F. Signs of Marine Ecosystem Rehabilitation Along the Romanian Black Sea Littoral Identified by Zooplankton Indicator after Cercet. Mar. 2004, 35, 87–108. [Google Scholar]
- Timofte, F. Contribuții La Evaluarea Stării Ecologice Actuale a Populațiilor Zooplanctonice Din Apele Românești Ale Mării Negre; Ex Ponto: Constanta, Romania, 2017. [Google Scholar]
- Băcescu, M.; Gomoiu, M.T.; Bodeanu, N.; Petran, A.; Muller, G.I.; Chirilă, V. Dinamica Populațiilor Animale Și Vegetale Din Zona Nisipurilor Fine de La. Nord de Constanța În Condițiile Anilor 1962–1965; Academiei Republicii SocialIste România: Bucharest, Romania, 1967. [Google Scholar]
- Hembre, L.K.; Megard, R.O. Seasonal and Diel Patchiness of a Daphnia Population: An Acoustic Analysis. Limnol. Ocean. 2003, 48, 2221–2233. [Google Scholar] [CrossRef]
- Campbell, M.D.; Schoeman, D.S.; Venables, W.; Abu-Alhaija, R.; Batten, S.D.; Chiba, S.; Coman, F.; Davies, C.H.; Edwards, M.; Eriksen, R.S.; et al. Testing Bergmann’s Rule in Marine Copepods. Ecography 2021, 44, 1283–1295. [Google Scholar] [CrossRef]
- Ratnarajah, L.; Abu-Alhaija, R.; Atkinson, A.; Batten, S.; Bax, N.J.; Bernard, K.S.; Canonico, G.; Cornils, A.; Everett, J.D.; Grigoratou, M.; et al. Monitoring and Modelling Marine Zooplankton in a Changing Climate. Nat. Commun. 2023, 14, 564. [Google Scholar] [CrossRef]
- Brandão, M.C.; Benedetti, F.; Martini, S.; Soviadan, Y.D.; Irisson, J.-O.; Romagnan, J.-B.; Elineau, A.; Desnos, C.; Jalabert, L.; Freire, A.S.; et al. Macroscale Patterns of Oceanic Zooplankton Composition and Size Structure. Sci. Rep. 2021, 11, 15714. [Google Scholar] [CrossRef]
- Brun, P.; Stamieszkin, K.; Visser, A.W.; Licandro, P.; Payne, M.R.; Kiørboe, T. Climate Change Has Altered Zooplankton-Fuelled Carbon Export in the North Atlantic. Nat. Ecol. Evol. 2019, 3, 416–423. [Google Scholar] [CrossRef]
- Figueroa, D.F. Environmental Forcing on Zooplankton Distribution in the Coastal Waters of the Galápagos Islands: Spatial and Seasonal Patterns in the Copepod Community Structure. Mar. Ecol. Prog. Ser. 2021, 661, 49–69. [Google Scholar] [CrossRef]
- Abdulwahab, S.; Rabee, A.M. Ecological Factors Affecting the Distribution of the Zooplankton Community in the Tigris River at Baghdad Region, Iraq. Egypt. J. Aquat. Res. 2015, 41, 187–196. [Google Scholar] [CrossRef]
- Sinu, J.V.; Ajimila, B. Quantitative Composition, Distribution and Abundance of Zooplankton Communities in Relation to Physico-Chemical Parameters from Selected Beaches of Alappuzha in Arabian Sea, Southwest Coast of India. Total Environ. Res. 2023, 7, 100054. [Google Scholar] [CrossRef]
- Evans, L.E.; Hirst, A.G.; Kratina, P.; Beaugrand, G. Temperature-mediated Changes in Zooplankton Body Size: Large Scale Temporal and Spatial Analysis. Ecography 2020, 43, 581–590. [Google Scholar] [CrossRef]
- Fonda Umani, S. Noctiluca Scintillans MACARTNEY in the Northern Adriatic Sea: Long-Term Dynamics, Relationships with Temperature and Eutrophication, and Role in the Food Web. J. Plankton Res. 2004, 26, 545–561. [Google Scholar] [CrossRef]
- Elbrächter, M. and Q. Z. Aspects of Noctiluca (Dinophyceae) Population Dynamics, Physiological Ecology of Harmful Algal Blooms; Anderson, D.M., Cembella, A.D., Hallegraeff, G.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Huang, C.; Qi, Y. The Abundance Cycle and Influence Factors on Red Tide Phenomena of Noctiluca scintillans (Dinophyceae) in Dapeng Bay, the South China Sea. J. Plankton Res. 1997, 19, 303–318. [Google Scholar] [CrossRef]
- Tada1, K.; Plthakpol1, S.; Montani, S. Seasonal Variation in the Abundance of Noctiluca scintillans in the Seto Inland Sea, Japan. Plankton Biol. Ecol. 2004, 51, 7–14. [Google Scholar]
- Miyaguchi, H.; Fujiki, T.; Kikuchi, T.; Kuwahara, V.S.; Toda, T. Relationship between the Bloom of Noctiluca Scintillans and Environmental Factors in the Coastal Waters of Sagami Bay, Japan. J. Plankton Res. 2006, 28, 313–324. [Google Scholar] [CrossRef]
- Turkoglu, M. Red Tides of the Dinoflagellate Noctiluca scintillans Associated with Eutrophication in the Sea of Marmara (the Dardanelles, Turkey). Oceanologia 2013, 55, 709–732. [Google Scholar] [CrossRef]
- Weinstock, J.B.; Vargas, L.; Collin, R. Zooplankton Abundance Reflects Oxygen Concentration and Dissolved Organic Matter in a Seasonally Hypoxic Estuary. J. Mar. Sci. Eng. 2022, 10, 427. [Google Scholar] [CrossRef]
- Kiko, R.; Hauss, H. On the Estimation of Zooplankton-Mediated Active Fluxes in Oxygen Minimum Zone Regions. Front. Mar. Sci. 2019, 6, 741. [Google Scholar] [CrossRef]
- Glibert, P.M. Harmful Algae at the Complex Nexus of Eutrophication and Climate Change. Harmful Algae 2020, 91, 101583. [Google Scholar] [CrossRef] [PubMed]
- Bairagi, N.; Saha, S.; Chaudhuri, S.; Dana, S.K. Zooplankton Selectivity and Nutritional Value of Phytoplankton Influences a Rich Variety of Dynamics in a Plankton Population Model. Phys. Rev. E 2019, 99, 012406. [Google Scholar] [CrossRef]
- Wei, Y.; Ding, D.; Gu, T.; Jiang, T.; Qu, K.; Sun, J.; Cui, Z. Different Responses of Phytoplankton and Zooplankton Communities to Current Changing Coastal Environments. Environ. Res. 2022, 215, 114426. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, H.; Wang, Z.; Huang, T.; Tian, W.; Huang, H. Responses of Zooplankton Community Pattern to Environmental Factors along the Salinity Gradient in a Seagoing River in Tianjin, China. Microorganisms 2023, 11, 1638. [Google Scholar] [CrossRef]
- Kovalev, A.V.; Mazzocchi, M.G. Seasonal Changes in the Composition and Abundance of Zooplankton in the Seas of the Mediterranean Basin. Turk. J. Zool. 2003, 27, 205–219. [Google Scholar]
- Dippner, J.W.; Hänninen, J.; Kuosa, H.; Vuorinen, I. The Influence of Climate Variability on Zooplankton Abundance in the Northern Baltic Archipelago Sea (SW Finland). ICES J. Mar. Sci. 2001, 58, 569–578. [Google Scholar] [CrossRef]














| Variables | Categories | Counts | Frequencies | Rel. Frequency per Category (%) | Taxonomic Groups |
|---|---|---|---|---|---|
| Season | Cold | 1314 | 1314 | 18.1 | |
| Warm | 5940 | 5940 | 81.9 | ||
| Taxa | Acartia clausi | 379 | 379 | 5.2 | Copepoda |
| Balanus | 378 | 378 | 5.2 | Meroplankton | |
| Bivalvia | 378 | 378 | 5.2 | Meroplankton | |
| Bosmina longirostris | 123 | 123 | 1.7 | Cladocera | |
| Calanus euxinus | 364 | 364 | 5.0 | Copepoda | |
| Centropages ponticus | 334 | 334 | 4.6 | Copepoda | |
| Chydorus sphaericus | 47 | 47 | 0.6 | Cladocera | |
| Cyclops sp. | 127 | 127 | 1.8 | Copepoda | |
| Daphnia longispina | 42 | 42 | 0.6 | Cladocera | |
| Decapoda | 249 | 249 | 3.4 | Meroplankton | |
| Diaphanosoma brachyurum | 36 | 36 | 0.5 | Cladocera | |
| Evadne spinifera | 179 | 179 | 2.5 | Cladocera | |
| Gastropoda | 377 | 377 | 5.2 | Meroplankton | |
| Harpacticoida | 350 | 350 | 4.8 | Copepoda | |
| Mesopodopsis slabberi | 202 | 202 | 2.8 | Other groups | |
| Noctiluca scintillans | 379 | 379 | 5.2 | Non-fodder | |
| Oikopleura dioica | 378 | 378 | 5.2 | Other groups | |
| Oithona davisae | 158 | 158 | 2.2 | Copepoda | |
| Oithona similis | 377 | 377 | 5.2 | Copepoda | |
| Paracalanus parvus | 378 | 378 | 5.2 | Copepoda | |
| Parasagitta setosa | 377 | 377 | 5.2 | Other groups | |
| Penilia avirostris | 305 | 305 | 4.2 | Cladocera | |
| Pleopis polyphemoides | 378 | 378 | 5.2 | Cladocera | |
| Podon sp. | 4 | 4 | 0.1 | Cladocera | |
| Polychaeta | 378 | 378 | 5.2 | Meroplankton | |
| Pseudevadne tergestina | 199 | 199 | 2.7 | Cladocera | |
| Pseudocalanus elongatus | 378 | 378 | 5.2 | Copepoda |
| Warm | ||||||
| Average similarity: 96.14 | ||||||
| Taxonomic group | Av. Abund. | Av. Sim. | Sim./SD | Contrib. % | Cum. % | |
| Copepoda | 2.69 | 27.36 | 27.5 | 28.46 | 28.46 | |
| Meroplankton | 2.16 | 22.32 | 19.19 | 23.21 | 51.67 | |
| Cladocera | 2.05 | 20.49 | 11.8 | 21.32 | 72.99 | |
| Cold | ||||||
| Average similarity: 96.83 | ||||||
| Taxonomic group | Av. Abund. | Av. Sim. | Sim./SD | Contrib. % | Cum. % | |
| Copepoda | 2.54 | 28.35 | 18.77 | 29.27 | 29.27 | |
| Meroplankton | 2 | 23.9 | 35.29 | 24.68 | 53.95 | |
| Other groups | 1.73 | 20.69 | 35.29 | 21.37 | 75.32 | |
| Groups Warm and Cold | ||||||
| Average dissimilarity = 8.85 | ||||||
| Warm | Cold | |||||
| Taxonomic group | Av. Abund. | Av. Abund. | Av. Diss. | Diss./SD | Contrib. % | Cum. % |
| Cladocera | 2.05 | 1.1 | 5.33 | 3.45 | 60.16 | 60.16 |
| Copepoda | 2.69 | 2.54 | 1.44 | 1.29 | 16.24 | 76.4 |
| Year | Species Richness (S) | Shannon–Weiner Index (H′) | Pielou’s Evenness Index (J′) | Season |
|---|---|---|---|---|
| 2013 | 25 | 1.8 | 0.6 | Warm |
| 2014 | 18 | 1.4 | 0.5 | Warm |
| 2015 | 18 | 1.0 | 0.4 | Warm |
| 2016 | 19 | 1.9 | 0.7 | Warm |
| 2017 | 18 | 1.2 | 0.4 | Warm |
| 2018 | 21 | 2.3 | 0.8 | Warm |
| 2019 | 25 | 2.0 | 0.6 | Warm |
| 2020 | 23 | 1.6 | 0.5 | Warm |
| 2014 | 16 | 1.5 | 0.5 | Cold |
| 2016 | 15 | 1.3 | 0.5 | Cold |
| 2017 | 18 | 1.7 | 0.6 | Cold |
| F1 | F2 | F3 | F4 | |
|---|---|---|---|---|
| T (°C) | −0.017 | 0.512 | 0.506 | −0.378 |
| S (‰) | −0.704 | 0.887 | −0.415 | −0.400 |
| PO4 (µM) | −0.180 | −0.311 | 0.236 | −0.372 |
| SiO4 (µM) | −0.452 | 0.096 | −0.394 | −0.150 |
| NO2 (µM) | 0.333 | 0.455 | 0.126 | 0.071 |
| NO3 (µM) | −0.506 | −0.649 | 0.262 | −0.207 |
| NH4 (µM) | 0.094 | 0.156 | −0.161 | −0.046 |
| O2 (µM) | 0.258 | 0.363 | −0.459 | −0.887 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bișinicu, E.; Lazăr, L.; Timofte, F. Dynamics of Zooplankton along the Romanian Black Sea Coastline: Temporal Variation, Community Structure, and Environmental Drivers. Diversity 2023, 15, 1024. https://doi.org/10.3390/d15091024
Bișinicu E, Lazăr L, Timofte F. Dynamics of Zooplankton along the Romanian Black Sea Coastline: Temporal Variation, Community Structure, and Environmental Drivers. Diversity. 2023; 15(9):1024. https://doi.org/10.3390/d15091024
Chicago/Turabian StyleBișinicu, Elena, Luminița Lazăr, and Florin Timofte. 2023. "Dynamics of Zooplankton along the Romanian Black Sea Coastline: Temporal Variation, Community Structure, and Environmental Drivers" Diversity 15, no. 9: 1024. https://doi.org/10.3390/d15091024
APA StyleBișinicu, E., Lazăr, L., & Timofte, F. (2023). Dynamics of Zooplankton along the Romanian Black Sea Coastline: Temporal Variation, Community Structure, and Environmental Drivers. Diversity, 15(9), 1024. https://doi.org/10.3390/d15091024